Building an Atom Worksheet Answers Key
Understanding the Structure of an Atom
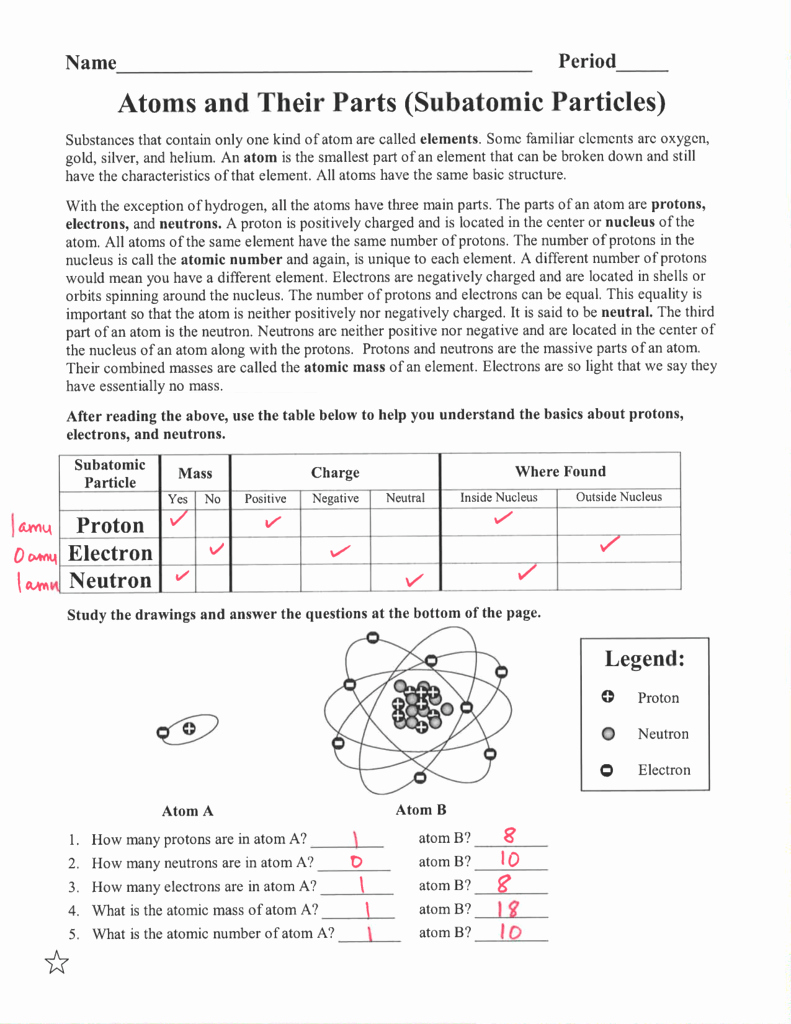
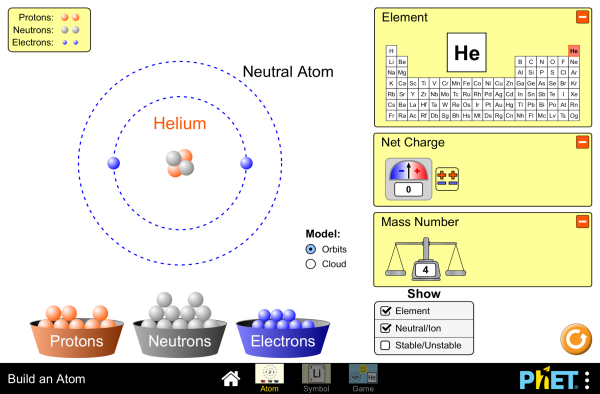
The atom is the fundamental building block of matter, and understanding its structure is crucial for chemistry and physics. The atom consists of three main parts: protons, neutrons, and electrons.
🔍 Note: The atom is incredibly small, with a radius of about 1-3 angstroms (Å).
Protons, Neutrons, and Electrons: What's the Difference?
- Protons: Positively charged particles found in the nucleus (center) of the atom.
- Neutrons: Particles with no charge that are also found in the nucleus.
- Electrons: Negatively charged particles that orbit the nucleus.
How to Determine the Number of Protons, Neutrons, and Electrons in an Atom
To determine the number of protons, neutrons, and electrons in an atom, you need to know the atomic number and mass number of the element.
- Atomic Number: The number of protons in an atom’s nucleus, which determines the element of an atom.
- Mass Number: The total number of protons and neutrons in an atom’s nucleus.
Calculating the Number of Neutrons
To calculate the number of neutrons in an atom, subtract the atomic number from the mass number.
| Atomic Number | Mass Number | Number of Neutrons |
|---|---|---|
| 6 (Carbon) | 12 | 6 |
| 8 (Oxygen) | 16 | 8 |
Electron Configuration: A Key to Understanding Chemical Properties
Electron configuration is the arrangement of electrons in an atom, which determines an element’s chemical properties.
- Energy Levels: The regions around the nucleus where electrons are found.
- Electron Shells: The energy levels that electrons occupy.
Understanding Electron Configuration Notation
Electron configuration notation is a shorthand way of describing the arrangement of electrons in an atom.
| Element | Electron Configuration |
|---|---|
| Hydrogen | 1s1 |
| Helium | 1s2 |
Practice Problems: Building an Atom Worksheet Answers Key
| Problem | Answer |
|---|---|
| 1. What is the atomic number of oxygen? | 8 |
| 2. How many electrons are in a neutral carbon atom? | 6 |
| 3. What is the mass number of a carbon atom with 6 neutrons? | 12 |
| 4. Write the electron configuration for a fluorine atom. | 1s2 2s2 2p5 |
📝 Note: Practice problems are a great way to reinforce your understanding of atomic structure and electron configuration.
Conclusion
In conclusion, understanding the structure of an atom is crucial for chemistry and physics. By knowing the number of protons, neutrons, and electrons in an atom, you can determine an element’s chemical properties and electron configuration.
What is the difference between atomic number and mass number?
+
The atomic number is the number of protons in an atom’s nucleus, while the mass number is the total number of protons and neutrons.
How do I calculate the number of neutrons in an atom?
+
Subtract the atomic number from the mass number to calculate the number of neutrons.
What is electron configuration notation?
+
Electron configuration notation is a shorthand way of describing the arrangement of electrons in an atom.
Related Terms:
- Building an atom PhET simulation
- Build an atom PhET worksheet
- PhET Build a molecule