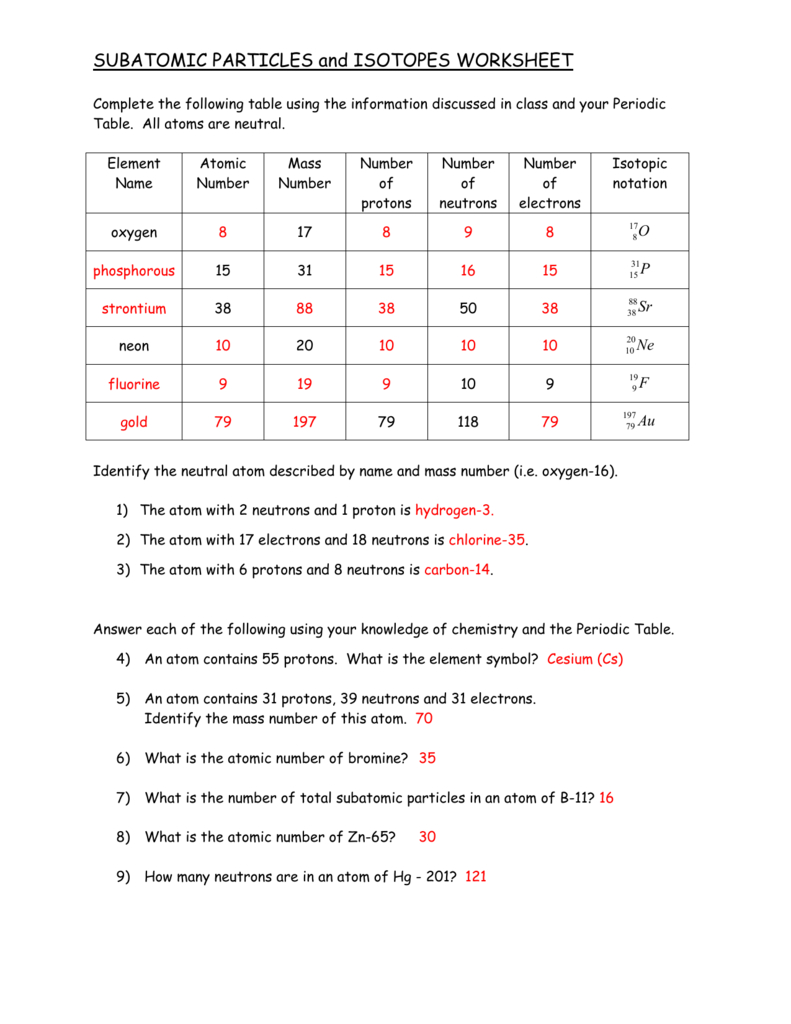
Subatomic Particles Worksheet
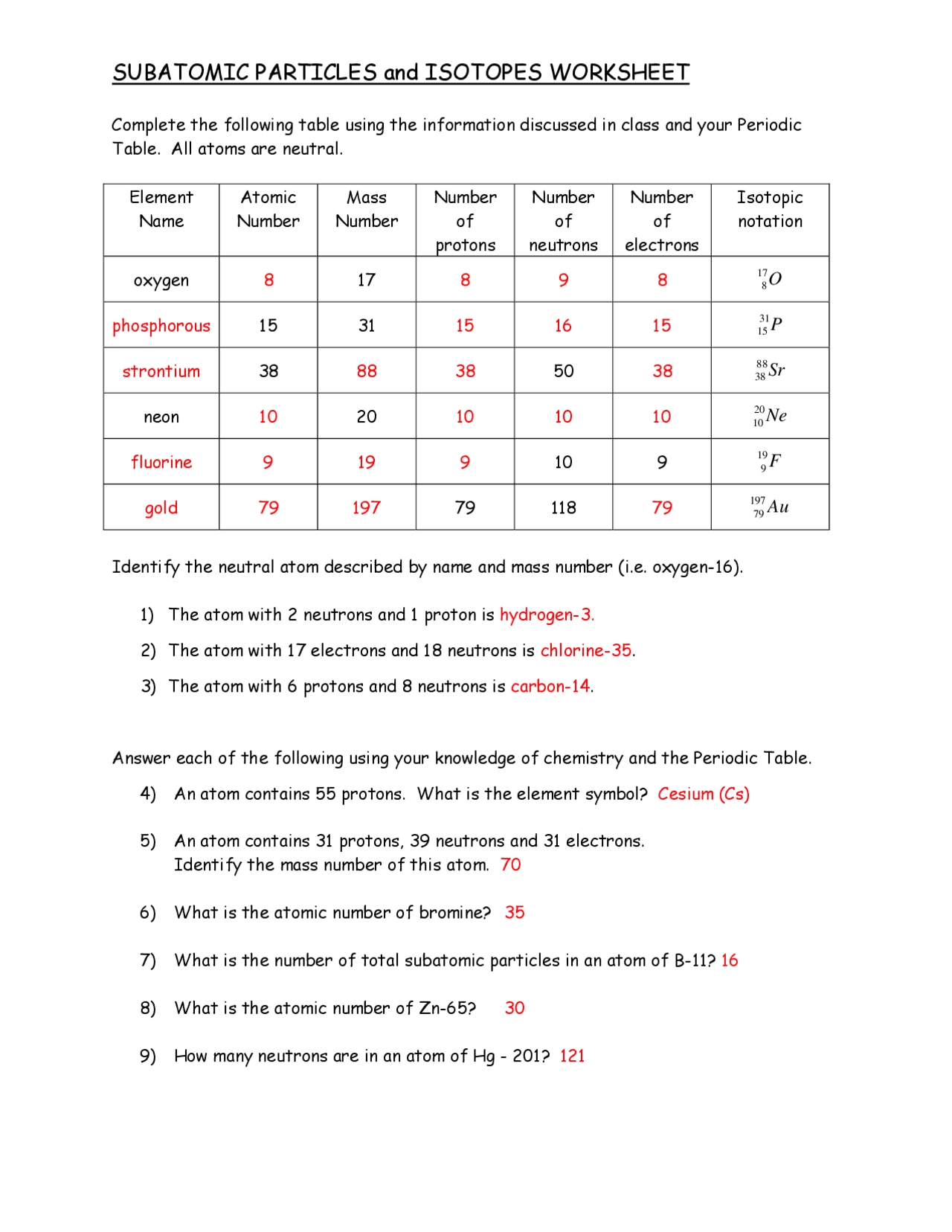
Understanding Subatomic Particles
The world of subatomic particles is a fascinating one, filled with tiny entities that make up the very building blocks of our universe. In this worksheet, we’ll delve into the wonderful world of protons, neutrons, electrons, and more.
The Structure of Atoms
Atoms are the smallest units of a chemical element, and they’re composed of three main subatomic particles: protons, neutrons, and electrons.
- Protons: These positively charged particles reside in the nucleus (center) of the atom. The number of protons in an atom determines the element of an atom, and each element has a unique number of protons in its atoms.
- Neutrons: These particles have no charge and are found in the nucleus along with protons. The number of neutrons in an atom can vary, leading to different isotopes (atoms of the same element with different numbers of neutrons) of an element.
- Electrons: These negatively charged particles orbit the nucleus of the atom. The number of electrons in an atom is equal to the number of protons, and this number determines the chemical properties of an element.
| Subatomic Particle | Charge | Location |
|---|---|---|
| Proton | Positive | Nucleus |
| Neutron | No charge | Nucleus |
| Electron | Negative | Orbiting the nucleus |
Other Subatomic Particles
In addition to protons, neutrons, and electrons, there are other subatomic particles that play important roles in the universe.
- Quarks: These particles are the building blocks of protons and neutrons. Quarks come in six “flavors” (up, down, charm, strange, top, and bottom) and three “colors” (red, green, and blue).
- Leptons: These particles are not part of the nucleus and do not participate in the strong nuclear force. Electrons are a type of lepton.
- Gluons: These particles are responsible for holding quarks together inside protons and neutrons.
- Photons: These particles are the quanta of light and are responsible for electromagnetic interactions.
Key Concepts to Remember
Here are some important points to keep in mind when studying subatomic particles:
- Atomic number: The number of protons in an atom’s nucleus, which determines the element of an atom.
- Mass number: The total number of protons and neutrons in an atom’s nucleus.
- Isotopes: Atoms of the same element with different numbers of neutrons.
- Ionization energy: The energy required to remove an electron from an atom.
🔍 Note: The atomic number of an element is the same as the number of electrons in a neutral atom of that element.
Practice Problems
Try solving these problems to test your understanding of subatomic particles:
- What is the charge of a proton?
- What is the location of an electron in an atom?
- What is the difference between an atom’s atomic number and mass number?
- What is the role of gluons in the strong nuclear force?
Conclusion
Subatomic particles are the tiny building blocks of our universe, and understanding their properties and behaviors is crucial for advancing our knowledge of physics and chemistry. By mastering the concepts of protons, neutrons, electrons, and other subatomic particles, you’ll be well on your way to unlocking the secrets of the atomic world.
What is the smallest unit of a chemical element?
+The smallest unit of a chemical element is an atom.
What determines the chemical properties of an element?
+The number of electrons in an atom determines the chemical properties of an element.
What is the role of quarks in the structure of atoms?
+Quarks are the building blocks of protons and neutrons, which make up the nucleus of an atom.
Related Terms:
- Subatomic Particles Worksheet PDF
- Subatomic particles worksheet With answers
- Subatomic particles worksheet PDF answers
- Counting subatomic particles Worksheet
- Subatomic particles chart
- Subatomic particles ions Worksheet