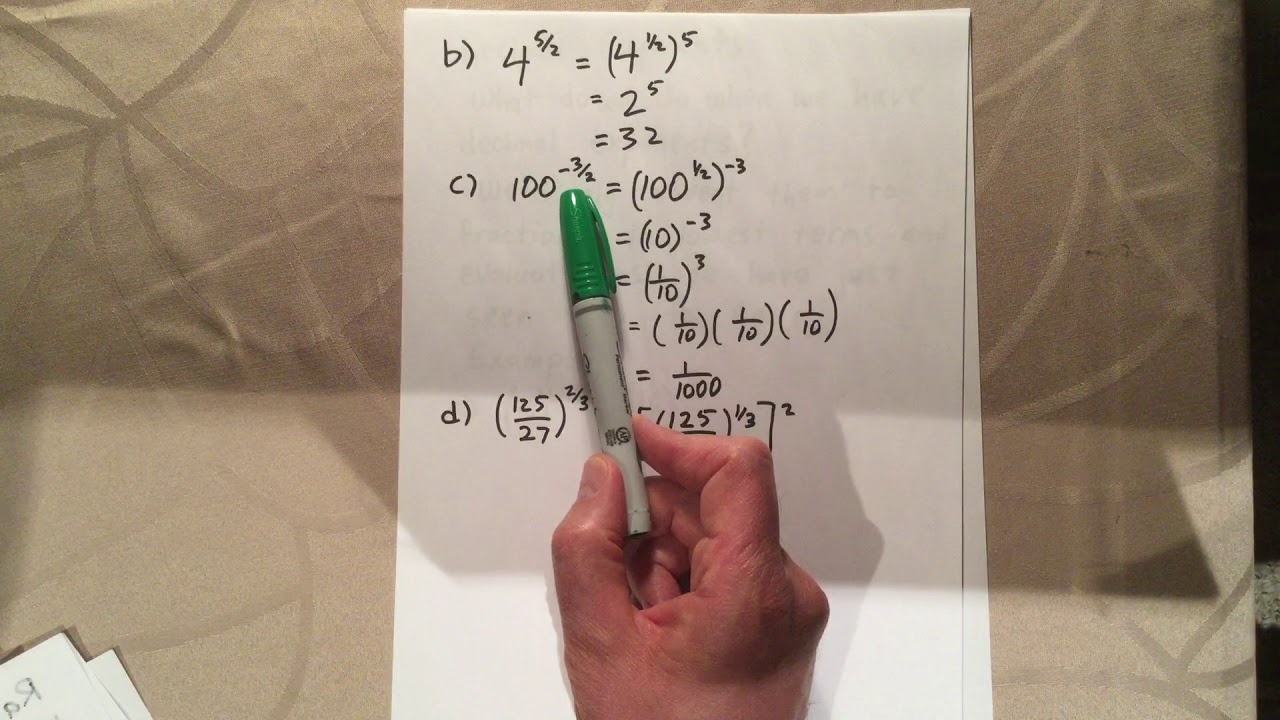Avogadro's Number and the Mole Worksheet Answers
Understanding Avogadro's Number and the Mole
Avogadro’s number and the mole are fundamental concepts in chemistry that help us understand the relationship between the amount of a substance and the number of particles it contains. In this article, we will delve into the world of Avogadro’s number and the mole, exploring their definitions, importance, and applications.
What is Avogadro's Number?
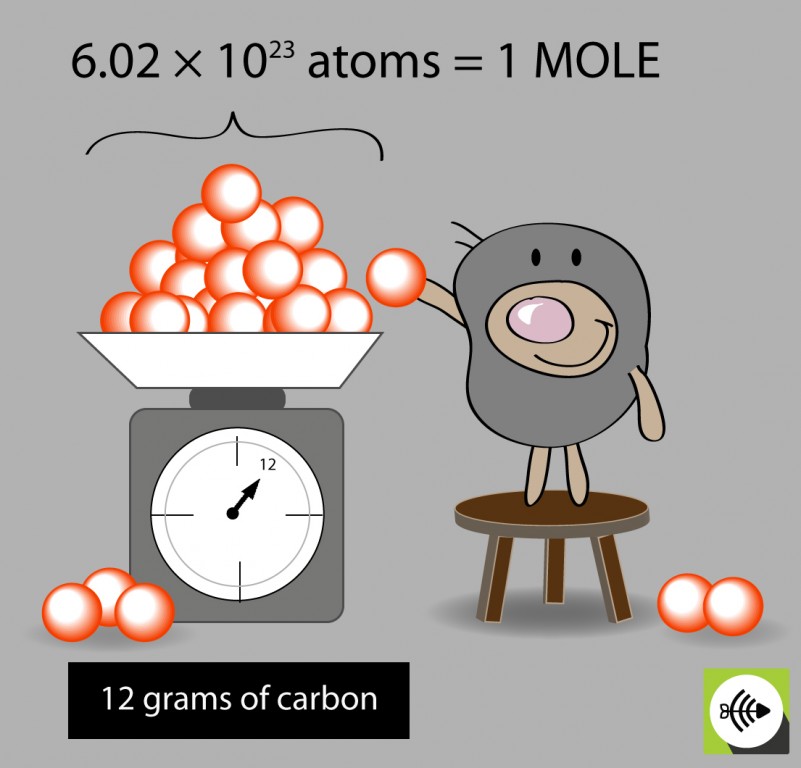
Avogadro’s number is a constant value that represents the number of particles (atoms or molecules) in one mole of a substance. It is named after the Italian scientist Amedeo Avogadro, who first proposed the idea in 1811. Avogadro’s number is equal to 6.022 x 10^23 particles.
What is the Mole?
The mole (mol) is the unit of measurement for the amount of a substance. It is defined as the amount of a substance that contains as many particles (atoms or molecules) as there are atoms in 0.012 kilograms of carbon-12. The mole is a convenient unit of measurement because it allows us to easily calculate the amount of a substance based on its molecular weight.
Relationship Between Avogadro's Number and the Mole
Avogadro’s number and the mole are closely related. One mole of a substance contains Avogadro’s number of particles (6.022 x 10^23). This means that if we know the molecular weight of a substance, we can calculate the number of moles it contains, and vice versa.
Calculating the Number of Moles
To calculate the number of moles of a substance, we can use the following formula:
moles = mass of substance / molecular weight
For example, if we have 10 grams of a substance with a molecular weight of 20 g/mol, we can calculate the number of moles as follows:
moles = 10 g / 20 g/mol = 0.5 mol
Calculating the Number of Particles
To calculate the number of particles (atoms or molecules) in a substance, we can use Avogadro’s number and the number of moles. The formula is:
number of particles = Avogadro’s number x number of moles
For example, if we have 0.5 mol of a substance, we can calculate the number of particles as follows:
number of particles = 6.022 x 10^23 x 0.5 = 3.011 x 10^23 particles
🔍 Note: When calculating the number of particles, make sure to use the correct units. Avogadro's number is typically expressed in units of particles per mole (6.022 x 10^23 particles/mol).
Applications of Avogadro's Number and the Mole
Avogadro’s number and the mole have numerous applications in chemistry and other fields. Some examples include:
- Chemical reactions: Understanding the mole concept is crucial for predicting the amount of reactants and products in chemical reactions.
- Stoichiometry: The mole concept is used to calculate the amount of substances required for a chemical reaction.
- Molecular weight: The mole concept is used to calculate the molecular weight of a substance.
- Gas laws: Avogadro’s number is used to calculate the volume of gases at standard temperature and pressure (STP).
Conclusion
In conclusion, Avogadro’s number and the mole are essential concepts in chemistry that help us understand the relationship between the amount of a substance and the number of particles it contains. By mastering these concepts, we can perform calculations and predict the behavior of substances in various chemical reactions and applications.
What is Avogadro’s number?
+
Avogadro’s number is a constant value that represents the number of particles (atoms or molecules) in one mole of a substance. It is equal to 6.022 x 10^23 particles.
What is the mole?
+
The mole (mol) is the unit of measurement for the amount of a substance. It is defined as the amount of a substance that contains as many particles (atoms or molecules) as there are atoms in 0.012 kilograms of carbon-12.
How do I calculate the number of moles?
+
To calculate the number of moles, use the formula: moles = mass of substance / molecular weight.
Related Terms:
- Mole/mass worksheet answers
- The Mole Worksheet chemistry answers
- Moles Worksheet answer key