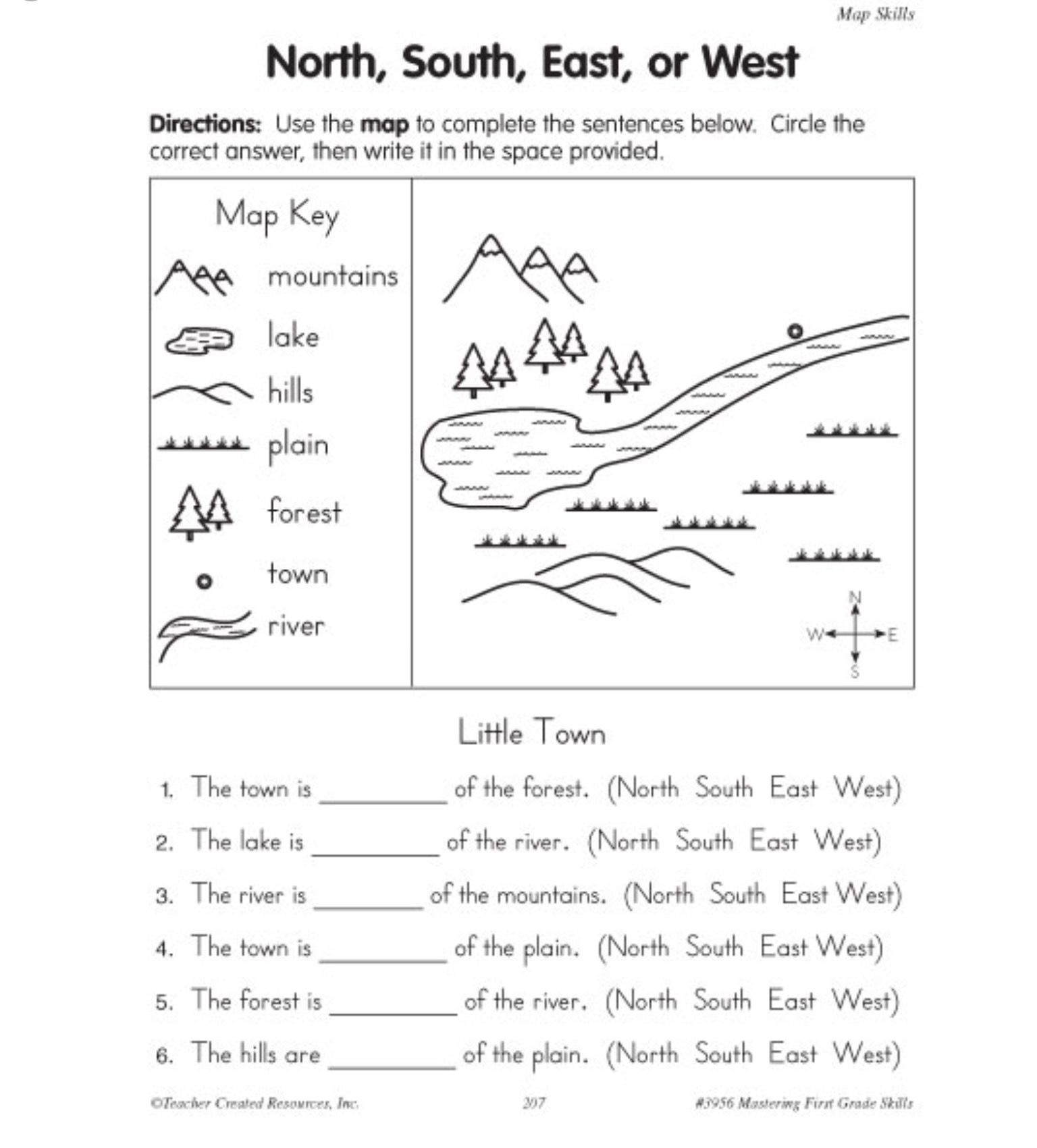
5 Essential Atom Structure Worksheet Answers
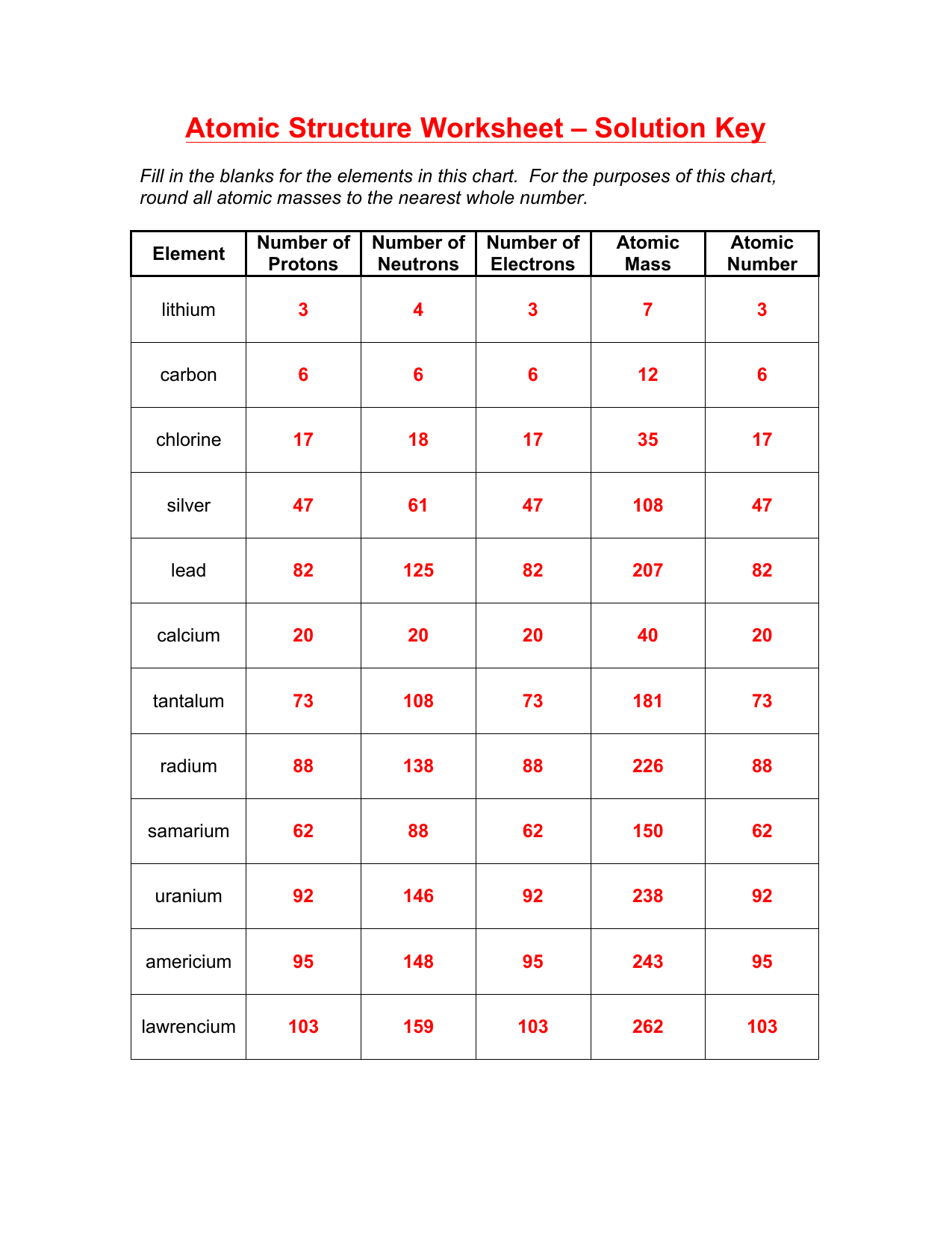
Understanding the Atom Structure: A Comprehensive Guide
The atom is the fundamental building block of matter, and understanding its structure is crucial in chemistry and physics. In this article, we will delve into the essential aspects of the atom structure, providing you with a comprehensive guide to enhance your knowledge. We will also cover 5 essential atom structure worksheet answers to help you grasp the concepts better.
The Basic Structure of an Atom
An atom consists of three main parts: protons, neutrons, and electrons.
- Protons: Positively charged particles that reside in the nucleus (center) of the atom.
- Neutrons: Particles with no charge that are also found in the nucleus.
- Electrons: Negatively charged particles that orbit the nucleus.
The number of protons in an atom determines the element of an atom, and each element has a unique number of protons in its atoms. The total number of protons and neutrons in an atom is known as the atomic mass.
Atomic Number and Mass Number
The atomic number of an atom is the number of protons present in its nucleus, while the mass number is the total number of protons and neutrons.
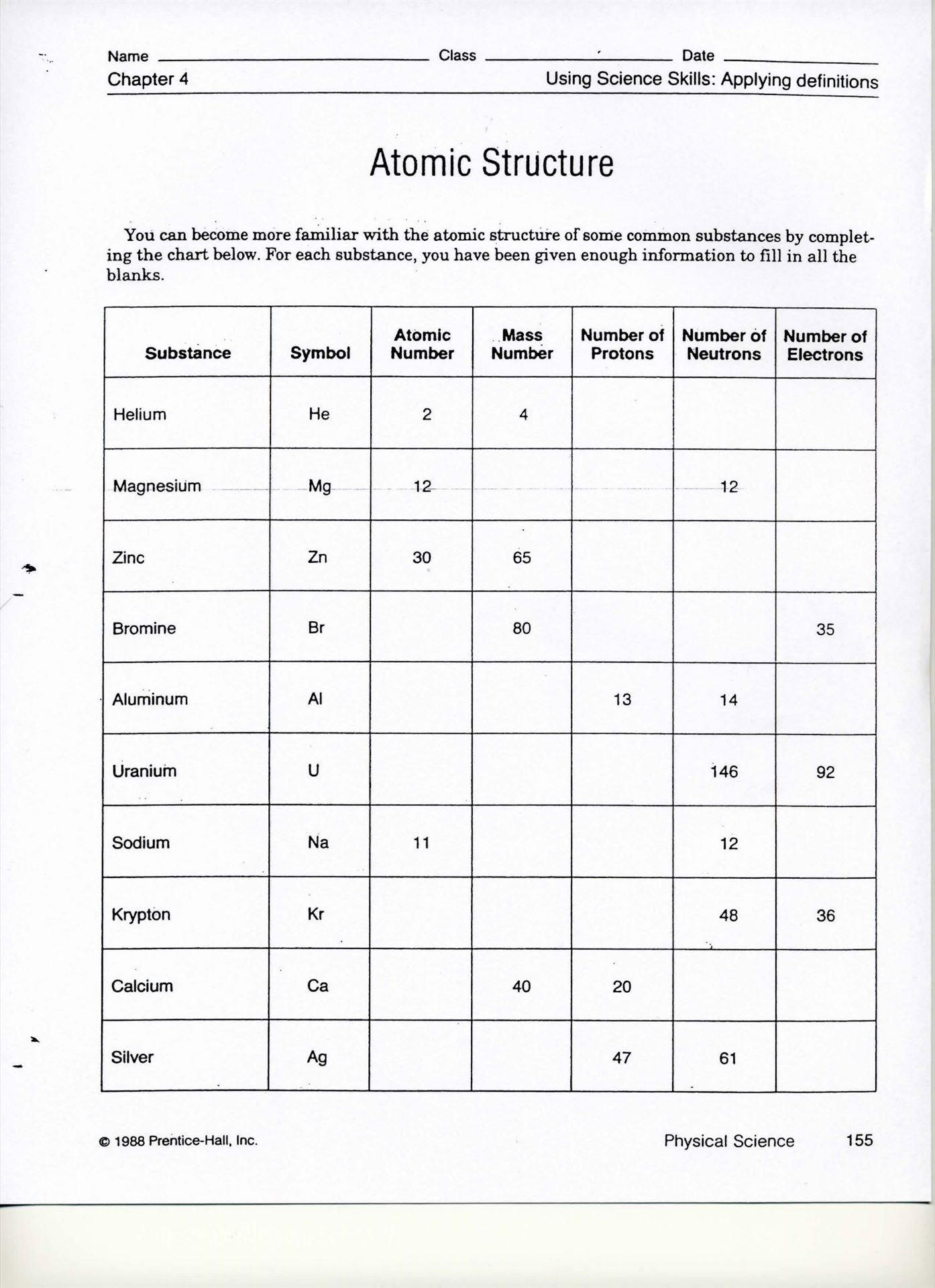
| Element | Atomic Number | Mass Number |
|---|---|---|
| Hydrogen | 1 | 1 |
| Helium | 2 | 4 |
| Oxygen | 8 | 16 |
Atomic Number vs. Mass Number:
- The atomic number is the number of protons in an atom.
- The mass number is the total number of protons and neutrons in an atom.
🔍 Note: The atomic number is unique to each element, while the mass number can vary for different isotopes of the same element.
Electron Shells and Energy Levels
Electrons occupy specific energy levels or shells around the nucleus. Each shell has a limited capacity, and electrons fill the lowest available energy levels first.
- Electron Shells: The regions around the nucleus where electrons are found.
- Energy Levels: The specific energy values that electrons occupy.
The first energy level (or shell) can hold up to 2 electrons, the second energy level can hold up to 8 electrons, and so on.
5 Essential Atom Structure Worksheet Answers
Here are five essential atom structure worksheet answers to help you reinforce your understanding:
- What is the atomic number of an element with 17 protons?
- Answer: 17
- What is the mass number of an atom with 6 protons and 6 neutrons?
- Answer: 12
- How many electrons can the first energy level hold?
- Answer: 2
- What is the symbol for the element with 8 protons?
- Answer: O (Oxygen)
- How many protons and neutrons does the element Carbon have?
- Answer: 6 protons and 6 neutrons
📝 Note: These answers are meant to provide a basic understanding of the atom structure. Make sure to practice more questions to reinforce your knowledge.
The atom structure is a fundamental concept in chemistry and physics, and understanding its basics is essential for further learning. By grasping the concepts of protons, neutrons, electrons, atomic number, mass number, electron shells, and energy levels, you will be well-equipped to tackle more advanced topics in science.
As you continue to learn about the atom structure, remember to practice regularly and reinforce your understanding with worksheets and exercises. With dedication and persistence, you will become proficient in understanding the intricacies of the atom structure.
In conclusion, understanding the atom structure is a vital part of science education. By mastering the basics of the atom structure, you will be able to grasp more advanced concepts and develop a deeper appreciation for the world of science.
What is the atomic number of an element?
+The atomic number of an element is the number of protons present in its atoms.
What is the mass number of an atom?
+The mass number of an atom is the total number of protons and neutrons present in its nucleus.
How many electrons can the first energy level hold?
+The first energy level can hold up to 2 electrons.
Related Terms:
- Atomic Structure Worksheet pdf