Master Oxidation Numbers in 5 Easy Steps
Mastering Oxidation Numbers: A Comprehensive Guide
Oxidation numbers are a crucial concept in chemistry, and understanding them is essential for any student or professional in the field. However, mastering oxidation numbers can be a daunting task, especially for those who are new to chemistry. In this article, we will break down the concept of oxidation numbers into 5 easy steps, making it easier for you to understand and apply them.
Step 1: Understanding the Basics
Before diving into the world of oxidation numbers, it’s essential to understand the basics of chemistry. Oxidation numbers are a way to keep track of the transfer of electrons during chemical reactions. They are assigned to atoms in a molecule or ion, and they can be positive, negative, or zero.
To understand oxidation numbers, you need to know the following:
- Atoms: The building blocks of matter, consisting of protons, neutrons, and electrons.
- Molecules: Groups of atoms bonded together.
- Ions: Atoms or molecules that have gained or lost electrons, resulting in a charge.
- Electrons: Negatively charged particles that orbit the nucleus of an atom.
Step 2: Assigning Oxidation Numbers
Assigning oxidation numbers is a straightforward process. Here are the rules to follow:
- Free elements: Atoms in their elemental form have an oxidation number of 0.
- Monatomic ions: Ions consisting of a single atom have an oxidation number equal to their charge.
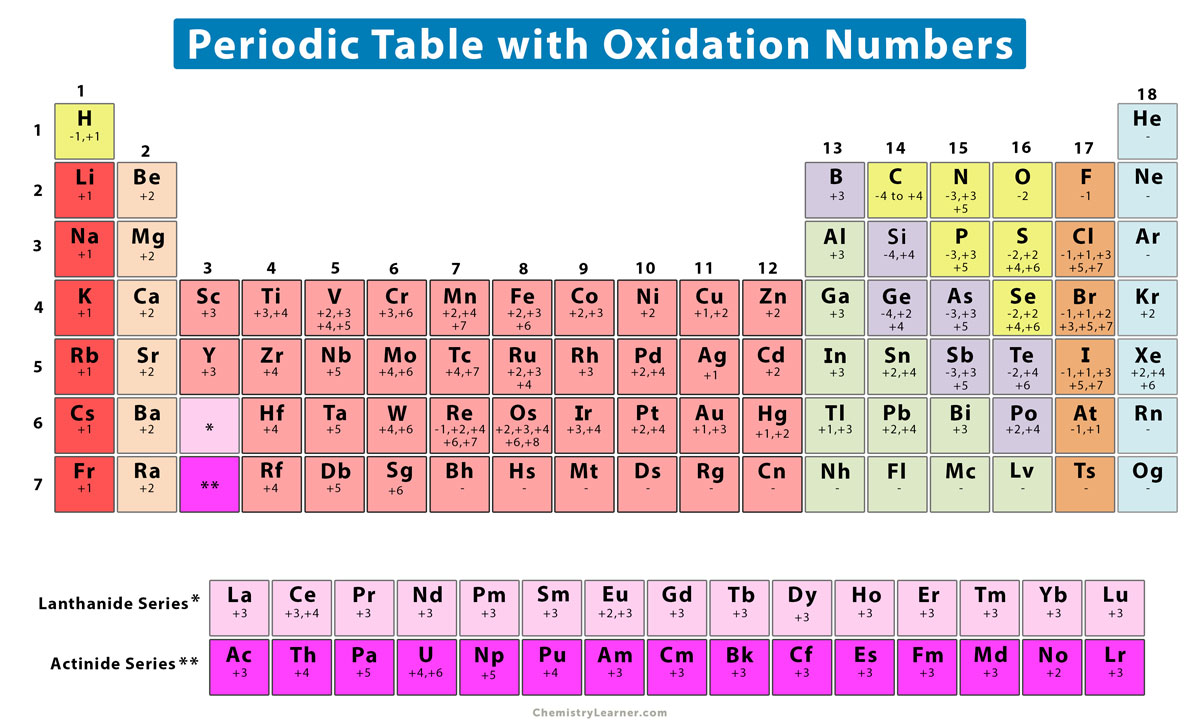
- Oxygen: Oxygen has an oxidation number of -2, except in peroxides, where it is -1.
- Hydrogen: Hydrogen has an oxidation number of +1, except in hydrides, where it is -1.
- Halogen: Halogens (F, Cl, Br, I) have an oxidation number of -1, except when bonded to oxygen or fluorine.
- Other elements: The oxidation number of other elements can be determined by looking at the charge on the ion or molecule.

| Element | Oxidation Number |
|---|---|
| Oxygen | -2 |
| Hydrogen | +1 |
| Halogen | -1 |
| Free elements | 0 |
Step 3: Applying Oxidation Numbers to Molecules
Now that you understand how to assign oxidation numbers, it’s time to apply them to molecules. Here are some examples:
- Water (H2O): Hydrogen has an oxidation number of +1, and oxygen has an oxidation number of -2. The sum of the oxidation numbers is 0.
- Carbon dioxide (CO2): Carbon has an oxidation number of +4, and oxygen has an oxidation number of -2. The sum of the oxidation numbers is 0.
When applying oxidation numbers to molecules, make sure to follow these rules:
- Sum of oxidation numbers: The sum of the oxidation numbers of all atoms in a molecule must be equal to the charge on the molecule.
- Neutral molecules: The sum of the oxidation numbers of all atoms in a neutral molecule must be 0.
Step 4: Determining Oxidation Numbers in Reactions
Oxidation numbers are essential in chemical reactions, as they help track the transfer of electrons. Here are some examples:
- Combustion reaction: 2CH4 + 3O2 → 2CO2 + 3H2O
- Methane (CH4) has an oxidation number of -4, and oxygen has an oxidation number of 0. The oxidation number of methane increases to +4 in carbon dioxide.
- Redox reaction: 2Fe + O2 → 2FeO
- Iron (Fe) has an oxidation number of 0, and oxygen has an oxidation number of 0. The oxidation number of iron increases to +2 in iron oxide.
When determining oxidation numbers in reactions, make sure to follow these rules:
- Oxidation: An increase in oxidation number indicates oxidation.
- Reduction: A decrease in oxidation number indicates reduction.
Step 5: Practice, Practice, Practice
The final step in mastering oxidation numbers is to practice, practice, practice! Here are some examples to get you started:
- Calculate the oxidation number of sulfur in sulfuric acid (H2SO4).
- Determine the oxidation number of nitrogen in ammonia (NH3).
- Assign oxidation numbers to the atoms in glucose (C6H12O6).
By following these 5 easy steps, you’ll become proficient in assigning oxidation numbers and applying them to molecules and reactions.
📝 Note: Oxidation numbers are a fundamental concept in chemistry, and mastering them takes time and practice. Don't be discouraged if you don't understand them immediately. Keep practicing, and you'll become proficient in no time!
In conclusion, oxidation numbers are a crucial concept in chemistry that helps track the transfer of electrons during chemical reactions. By following the 5 easy steps outlined in this article, you’ll become proficient in assigning oxidation numbers and applying them to molecules and reactions. Remember to practice, practice, practice, and you’ll master oxidation numbers in no time!
What is the oxidation number of oxygen in water?
+The oxidation number of oxygen in water is -2.
How do you determine the oxidation number of an atom in a molecule?
+The oxidation number of an atom in a molecule can be determined by looking at the charge on the ion or molecule and applying the rules outlined in this article.
What is the difference between oxidation and reduction?
+Oxidation is an increase in oxidation number, while reduction is a decrease in oxidation number.