Osmosis and Diffusion Worksheet for Biology Students
Understanding Osmosis and Diffusion: A Guide for Biology Students
As biology students, it’s essential to grasp the concepts of osmosis and diffusion, two fundamental processes that occur in living organisms. These processes play a crucial role in maintaining cellular homeostasis and facilitating the exchange of essential substances between cells and their environment. In this worksheet, we’ll delve into the world of osmosis and diffusion, exploring their definitions, differences, and significance in biological systems.
What is Diffusion?
Diffusion is the passive movement of particles from an area of higher concentration to an area of lower concentration. This process tends to equalize the concentration of substances within a given space. Diffusion occurs in all directions and is an essential mechanism for cells to obtain the necessary nutrients and eliminate waste products.
Key Characteristics of Diffusion:
- Random Movement: Particles move randomly, resulting in a uniform distribution.
- Passive Process: No energy is required for diffusion to occur.
- Equalizes Concentration: Concentration of substances becomes uniform throughout the space.
What is Osmosis?
Osmosis is a special type of diffusion that involves the movement of water molecules through a selectively permeable membrane. This process occurs when there is a concentration gradient of solutes across the membrane, resulting in the movement of water molecules from an area of high concentration to an area of low concentration.
Key Characteristics of Osmosis:
- Selective Permeability: Membrane allows certain substances to pass through while restricting others.
- Water Movement: Water molecules move through the membrane, influenced by the concentration gradient.
- Maintains Homeostasis: Osmosis helps regulate the balance of fluids within cells and tissues.
Differences between Osmosis and Diffusion
While both osmosis and diffusion involve the movement of particles, there are key differences between the two processes:
- Direction: Diffusion occurs in all directions, whereas osmosis occurs in one direction, from high to low concentration.
- Substance Movement: Diffusion involves the movement of various substances, whereas osmosis involves the movement of water molecules.
- Selectivity: Diffusion does not involve selective permeability, whereas osmosis occurs through a selectively permeable membrane.
💡 Note: Understanding the differences between osmosis and diffusion is crucial for grasping various biological concepts, including cellular transport and homeostasis.
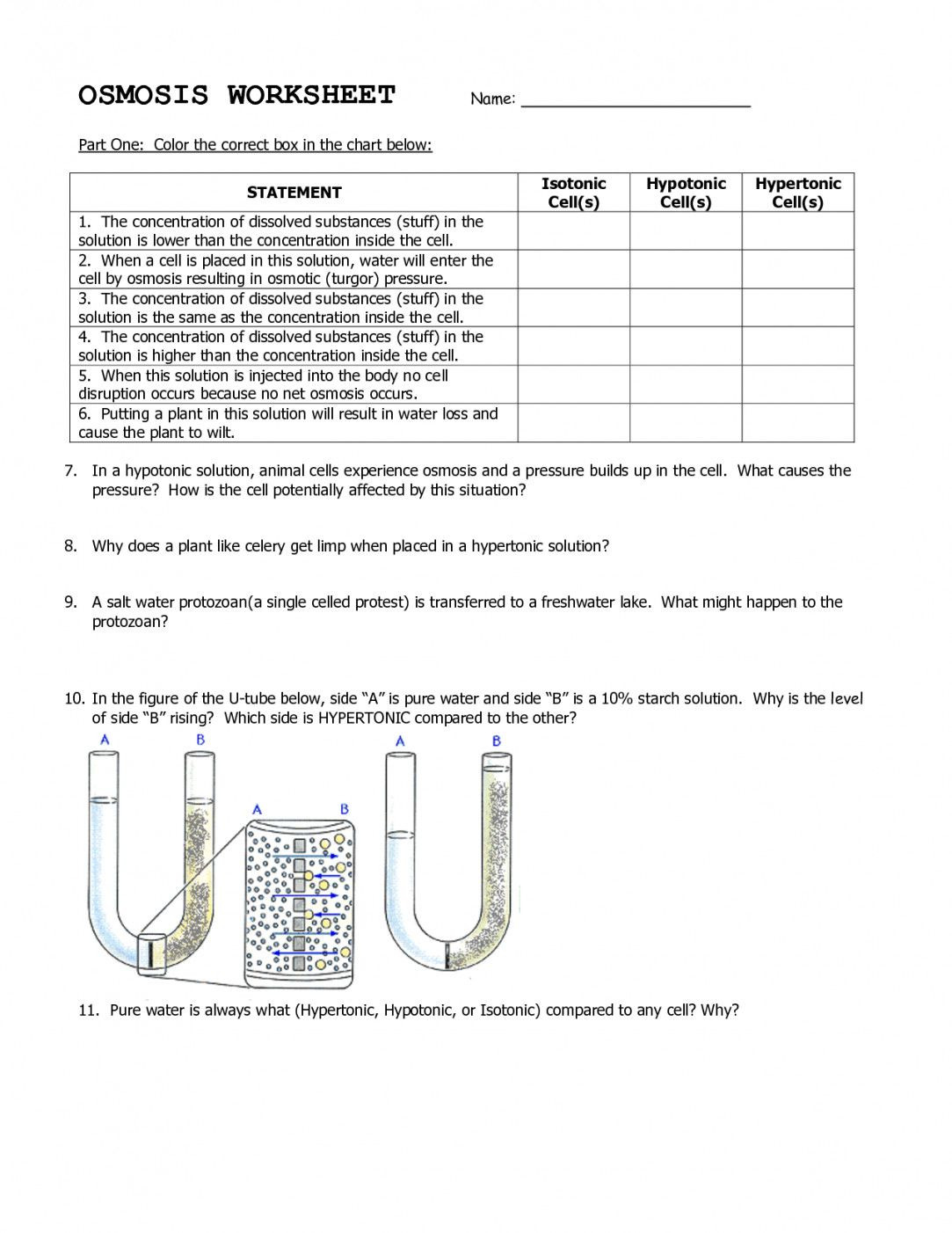
Types of Solutions and Their Effects on Cells
When discussing osmosis, it’s essential to understand the different types of solutions and their effects on cells:

| Solution Type | Concentration | Effect on Cells |
|---|---|---|
| Isotonic | Equal | No net movement of water molecules |
| Hypotonic | Lower | Water enters the cell, causing swelling |
| Hypertonic | Higher | Water leaves the cell, causing shrinkage |
📝 Note: The type of solution can significantly impact cellular structure and function, highlighting the importance of maintaining proper osmotic balance.
Biological Significance of Osmosis and Diffusion
Osmosis and diffusion play vital roles in various biological processes, including:
- Nutrient uptake and waste removal: Cells rely on diffusion to obtain essential nutrients and eliminate waste products.
- Cellular homeostasis: Osmosis helps maintain proper fluid balance within cells, ensuring optimal functioning.
- Transport across cell membranes: Both osmosis and diffusion facilitate the movement of substances across cell membranes.
By understanding the mechanisms of osmosis and diffusion, we can better appreciate the intricate processes that occur within living organisms.
As we conclude our exploration of osmosis and diffusion, remember that these processes are fundamental to life, allowing cells to maintain homeostasis and interact with their environment.
What is the primary difference between osmosis and diffusion?
+
Osmosis involves the movement of water molecules through a selectively permeable membrane, whereas diffusion involves the movement of various substances in any direction.
What type of solution would cause water to leave a cell?
+
A hypertonic solution would cause water to leave a cell, resulting in cell shrinkage.
Why is osmosis important for cellular homeostasis?
+
Osmosis helps maintain proper fluid balance within cells, ensuring optimal cellular functioning and preventing damage from excessive or inadequate fluid levels.