6 Easy Steps to Calculate Molarity
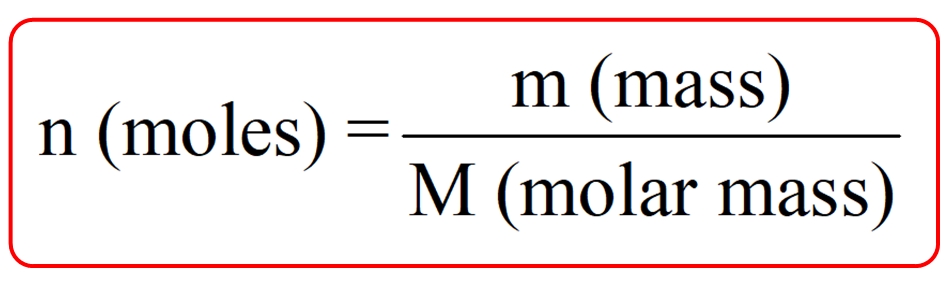
What is Molarity and Why is it Important?
Molarity is a measure of the concentration of a solution, defined as the number of moles of solute per liter of solution. It is a fundamental concept in chemistry and is widely used in various fields such as biology, medicine, and environmental science. Calculating molarity is crucial in determining the correct amount of a substance needed for a specific reaction or application.
Understanding the Formula for Molarity
The formula for calculating molarity is:
Molarity (M) = Number of Moles of Solute (n) / Volume of Solution (V) in Liters
M = n / V
Where:
- M is the molarity of the solution
- n is the number of moles of solute
- V is the volume of the solution in liters
Step 1: Determine the Number of Moles of Solute
To calculate the number of moles of solute, you need to know the molar mass of the substance and the amount of the substance in grams.
Number of Moles (n) = Mass of Solute (m) / Molar Mass (M)
For example, if you have 10 grams of sodium chloride (NaCl) with a molar mass of 58.44 g/mol, the number of moles would be:
n = 10 g / 58.44 g/mol = 0.171 mol
📝 Note: Make sure to use the correct molar mass for the substance you are working with.
Step 2: Determine the Volume of the Solution
To calculate the volume of the solution, you need to know the amount of solvent used and the density of the solvent.
Volume (V) = Mass of Solvent (m) / Density (ρ)
For example, if you have 100 grams of water with a density of 1 g/mL, the volume would be:
V = 100 g / 1 g/mL = 100 mL
To convert the volume from milliliters to liters, divide by 1000:
V = 100 mL / 1000 = 0.1 L
📝 Note: Make sure to use the correct density for the solvent you are working with.
Step 3: Calculate the Molarity
Now that you have the number of moles of solute and the volume of the solution, you can calculate the molarity using the formula:
M = n / V
Using the values from the previous steps:
M = 0.171 mol / 0.1 L = 1.71 M
Step 4: Check Your Units
Make sure to check your units to ensure that you are working with the correct units. Molarity is typically expressed in units of moles per liter (M).
Step 5: Calculate Molarity for Different Concentrations
To calculate molarity for different concentrations, you can use the same formula and adjust the number of moles of solute and the volume of the solution accordingly.
For example, if you want to calculate the molarity of a 2 M solution of sodium chloride, you would use:
n = 20 g / 58.44 g/mol = 0.342 mol
V = 100 mL / 1000 = 0.1 L
M = 0.342 mol / 0.1 L = 2 M
Step 6: Practice, Practice, Practice!
Calculating molarity is a skill that requires practice to master. Try calculating the molarity of different solutions using different concentrations and solutes.
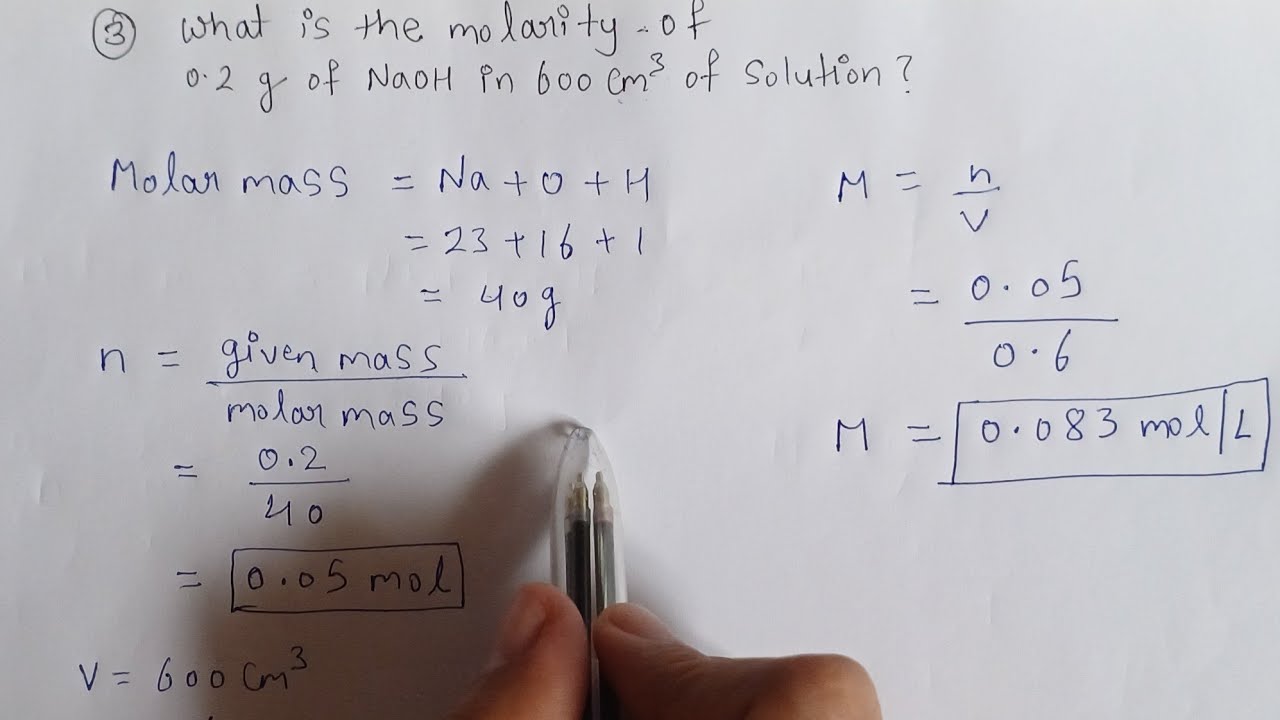
| Solute | Mass of Solute (g) | Volume of Solution (mL) | Molarity (M) |
|---|---|---|---|
| Sodium Chloride (NaCl) | 10 | 100 | 1.71 |
| Sodium Chloride (NaCl) | 20 | 100 | 2 |
| Sodium Chloride (NaCl) | 30 | 100 | 3 |
In conclusion, calculating molarity is a straightforward process that requires attention to detail and practice to master. By following these six easy steps, you can calculate the molarity of any solution and ensure that you are working with the correct concentrations for your experiments and applications.
What is the formula for calculating molarity?
+Molarity (M) = Number of Moles of Solute (n) / Volume of Solution (V) in Liters
How do I calculate the number of moles of solute?
+Number of Moles (n) = Mass of Solute (m) / Molar Mass (M)
What is the difference between molarity and concentration?
+Molarity is a measure of the concentration of a solution, while concentration is a more general term that can refer to any measure of the amount of substance in a solution.