Valence Electrons Worksheet Answers Simplified
Understanding Valence Electrons: A Simplified Approach
Valence electrons are the electrons in the outermost energy level of an atom. They play a crucial role in determining the chemical properties of an element, including its reactivity and ability to form compounds with other elements. In this blog post, we will delve into the world of valence electrons, exploring what they are, how to determine them, and their significance in chemistry.
What are Valence Electrons?
Valence electrons are the electrons in the outermost energy level of an atom, also known as the valence shell. The number of valence electrons in an atom determines its chemical reactivity and the types of bonds it can form with other atoms. Valence electrons are typically found in the s and p orbitals of an atom’s outermost energy level.
How to Determine Valence Electrons
Determining the number of valence electrons in an atom is relatively straightforward. Here are the steps:
- Identify the element’s atomic number, which represents the number of protons in the atom’s nucleus.
- Determine the element’s electron configuration, which shows how the electrons are arranged in the atom’s energy levels.
- Identify the outermost energy level, which is the valence shell.
- Count the number of electrons in the valence shell, which represents the number of valence electrons.
📝 Note: The number of valence electrons can also be determined by using the periodic table. Elements in the same group (vertical column) of the periodic table have the same number of valence electrons.
Valence Electrons and the Periodic Table
The periodic table is a powerful tool for determining the number of valence electrons in an atom. Elements in the same group (vertical column) of the periodic table have the same number of valence electrons. This is because elements in the same group have the same electron configuration in their outermost energy level.
| Group Number | Number of Valence Electrons |
|---|---|
| 1 (Alkali Metals) | 1 |
| 2 (Alkaline Earth Metals) | 2 |
| 13 (Boron Group) | 3 |
| 14 (Carbon Group) | 4 |
| 15 (Nitrogen Group) | 5 |
| 16 (Oxygen Group) | 6 |
| 17 (Halogens) | 7 |
| 18 (Noble Gases) | 8 |
Significance of Valence Electrons
Valence electrons play a crucial role in determining the chemical properties of an element. Here are some of the ways valence electrons impact an element’s chemistry:
- Reactivity: The number of valence electrons determines an element’s reactivity. Elements with a full outer energy level (8 valence electrons) are unreactive, while those with a partially filled outer energy level are more reactive.
- Bonding: Valence electrons participate in chemical bonding, which is the process of forming compounds with other elements. The number of valence electrons determines the types of bonds an element can form.
- Ionization Energy: The number of valence electrons affects an element’s ionization energy, which is the energy required to remove an electron from an atom.
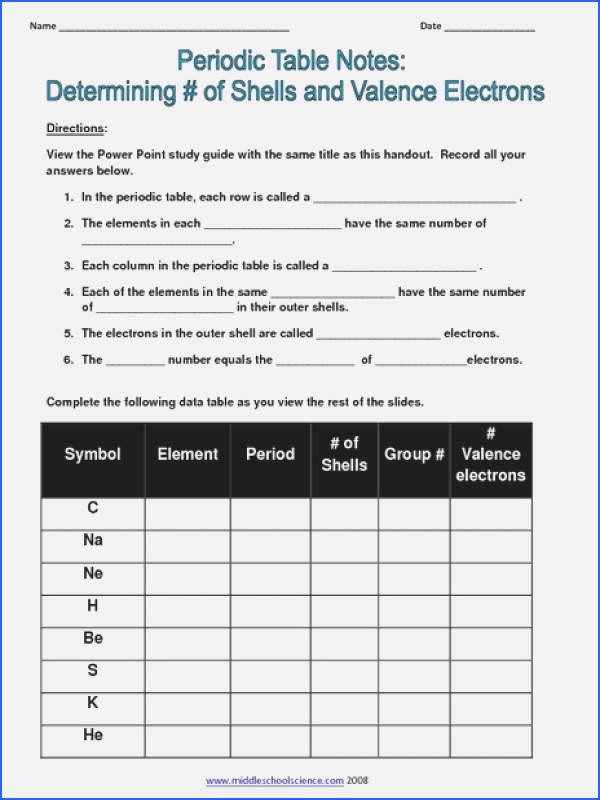
Valence Electrons Worksheet Answers
Here are the answers to a sample valence electrons worksheet:
- What is the number of valence electrons in sodium (Na)?
- Answer: 1
- What is the number of valence electrons in carbon ©?
- Answer: 4
- What is the number of valence electrons in oxygen (O)?
- Answer: 6
- What is the number of valence electrons in neon (Ne)?
- Answer: 8
📝 Note: These answers can be determined by using the periodic table or by counting the number of electrons in the outermost energy level of each element.
In conclusion, valence electrons are a fundamental concept in chemistry that determines an element’s chemical properties. By understanding how to determine valence electrons and their significance in chemistry, students can better appreciate the behavior of elements and their interactions with other elements.
What is the definition of valence electrons?
+Valence electrons are the electrons in the outermost energy level of an atom.
How do you determine the number of valence electrons in an atom?
+The number of valence electrons can be determined by identifying the element’s atomic number, determining the element’s electron configuration, identifying the outermost energy level, and counting the number of electrons in the valence shell.
What is the significance of valence electrons in chemistry?
+Valence electrons play a crucial role in determining the chemical properties of an element, including its reactivity, bonding, and ionization energy.
Related Terms:
- Valence electrons Worksheet Answers PDF
- Valence electrons worksheet pdf
- Valence electrons Practice worksheet
- Electron configuration chart Worksheet Answers
- Valence electrons sheet