Chemistry Unit Conversion Worksheet for Easy Calculations
Mastering Chemistry Unit Conversion with Ease
Chemistry unit conversion is an essential skill for any student or professional in the field of chemistry. It allows for the accurate calculation of quantities and concentrations, ensuring that experiments are conducted safely and effectively. In this article, we will explore the world of chemistry unit conversion, providing you with a comprehensive worksheet to practice and reinforce your skills.
Understanding the Basics of Chemistry Unit Conversion
Before diving into the worksheet, let’s review the fundamental concepts of chemistry unit conversion. Chemistry involves the measurement of various quantities, such as mass, volume, concentration, and temperature. Each of these quantities has its own set of units, and being able to convert between them is crucial.
Mass Units

| Unit | Abbreviation | Conversion Factor |
|---|---|---|
| Gram | g | 1 g = 0.001 kg |
| Kilogram | kg | 1 kg = 1000 g |
| Milligram | mg | 1 mg = 0.001 g |
Volume Units
| Unit | Abbreviation | Conversion Factor |
|---|---|---|
| Liter | L | 1 L = 1000 mL |
| Milliliter | mL | 1 mL = 0.001 L |
Concentration Units
| Unit | Abbreviation | Conversion Factor |
|---|---|---|
| Molarity | M | 1 M = 1 mol/L |
| Molality | m | 1 m = 1 mol/kg |
Chemistry Unit Conversion Worksheet
Now that we have reviewed the basics, it’s time to put your skills to the test. Complete the following worksheet to practice converting between different units.
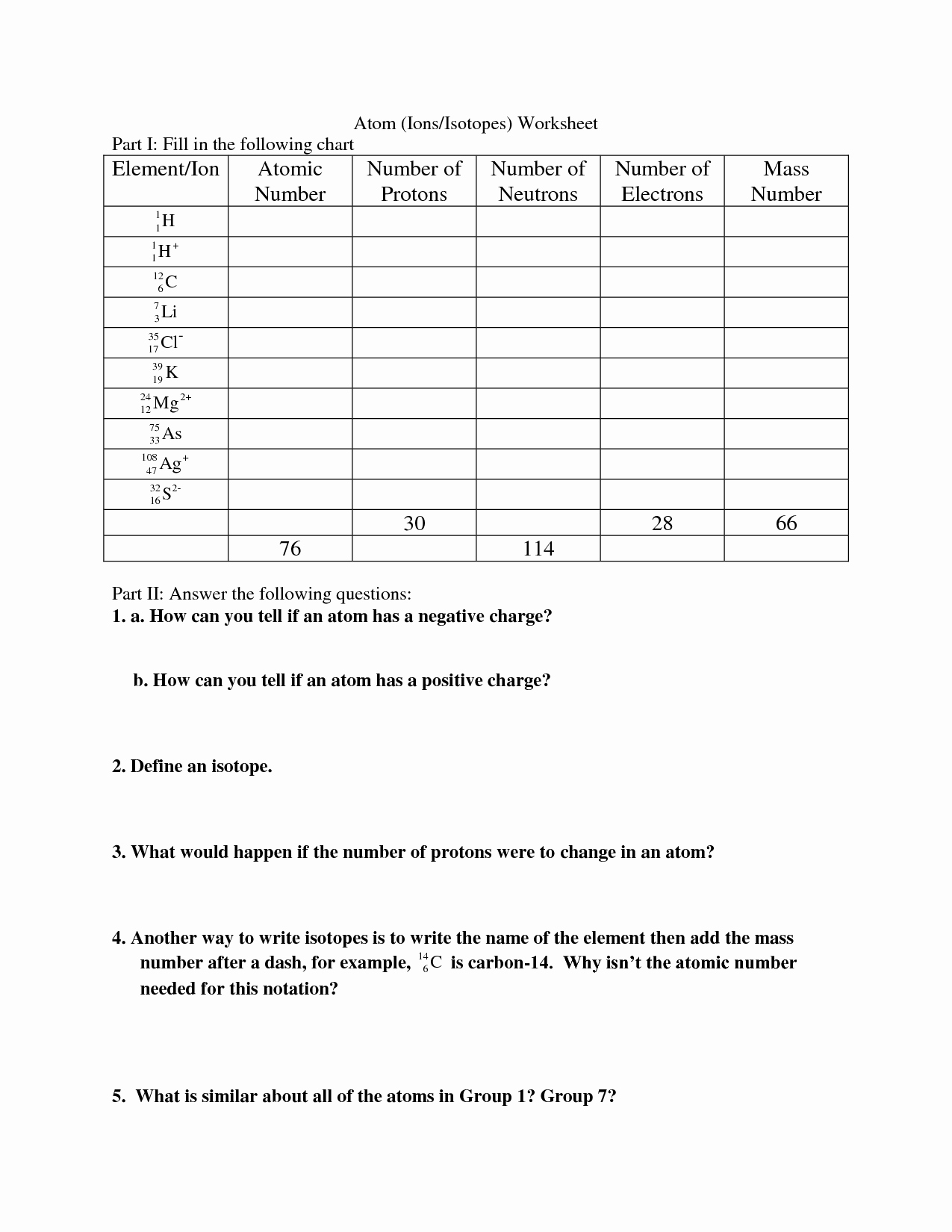
Section 1: Mass Conversion
- Convert 500 g to kilograms.
- Convert 2.5 kg to grams.
- Convert 100 mg to grams.
Section 2: Volume Conversion
- Convert 250 mL to liters.
- Convert 1.5 L to milliliters.
- Convert 500 mL to liters.
Section 3: Concentration Conversion
- Convert 2 M to molality (assuming a density of 1 g/mL).
- Convert 1.5 m to molarity (assuming a density of 1 g/mL).
- Convert 0.5 M to molality (assuming a density of 1.2 g/mL).
Section 4: Mixed Conversion
- Convert 250 g of a substance with a density of 2.5 g/mL to liters.
- Convert 1.2 L of a substance with a molarity of 3 M to grams.
- Convert 500 mg of a substance with a molality of 2 m to liters.
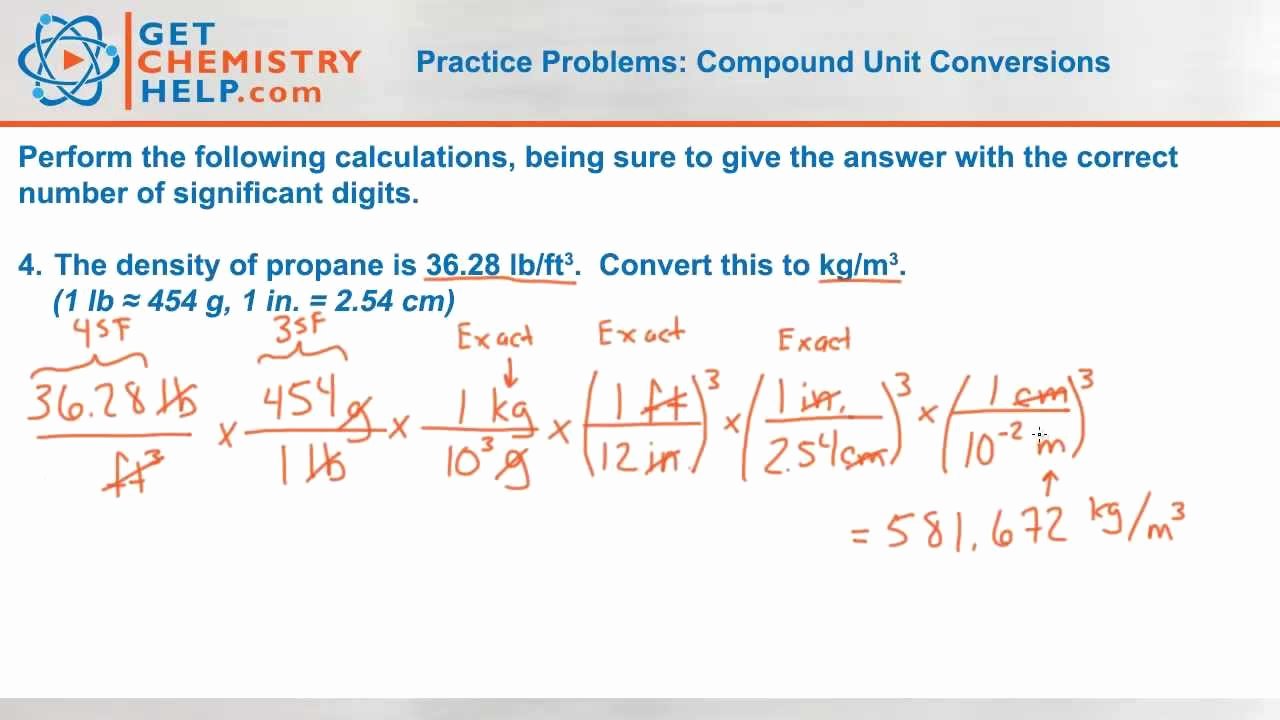
Tips and Tricks for Chemistry Unit Conversion
- Always check the units of the given quantities and the units of the desired quantities.
- Use conversion factors to convert between units.
- Be careful when converting between mass and volume units, as density may be involved.
- Practice, practice, practice! The more you practice, the more comfortable you will become with chemistry unit conversion.
📝 Note: When converting between units, make sure to cancel out the units correctly. For example, when converting from grams to kilograms, the "g" units will cancel out, leaving you with kilograms.
Conclusion
Mastering chemistry unit conversion is a vital skill for anyone working with chemicals. With practice and patience, you can become proficient in converting between different units. Remember to always check the units of the given quantities and the units of the desired quantities, and use conversion factors to convert between units. By following these tips and practicing with the worksheet provided, you will become a pro at chemistry unit conversion in no time.
What is the difference between mass and weight?
+Mass and weight are often used interchangeably, but they have distinct meanings. Mass refers to the amount of matter in an object, whereas weight refers to the force exerted on an object by gravity.
How do I convert between molarity and molality?
+To convert between molarity and molality, you need to know the density of the solution. Molarity is defined as the number of moles of solute per liter of solution, while molality is defined as the number of moles of solute per kilogram of solvent.
What is the significance of chemistry unit conversion in real-life scenarios?
+Chemistry unit conversion is crucial in various real-life scenarios, such as pharmaceutical manufacturing, food processing, and environmental monitoring. Accurate conversions ensure that the right quantities of chemicals are used, which can affect the safety and efficacy of products.
Related Terms:
- Unit conversion Worksheet PDF
- Chemistry conversion practice problems
- Metric unit conversions Worksheet