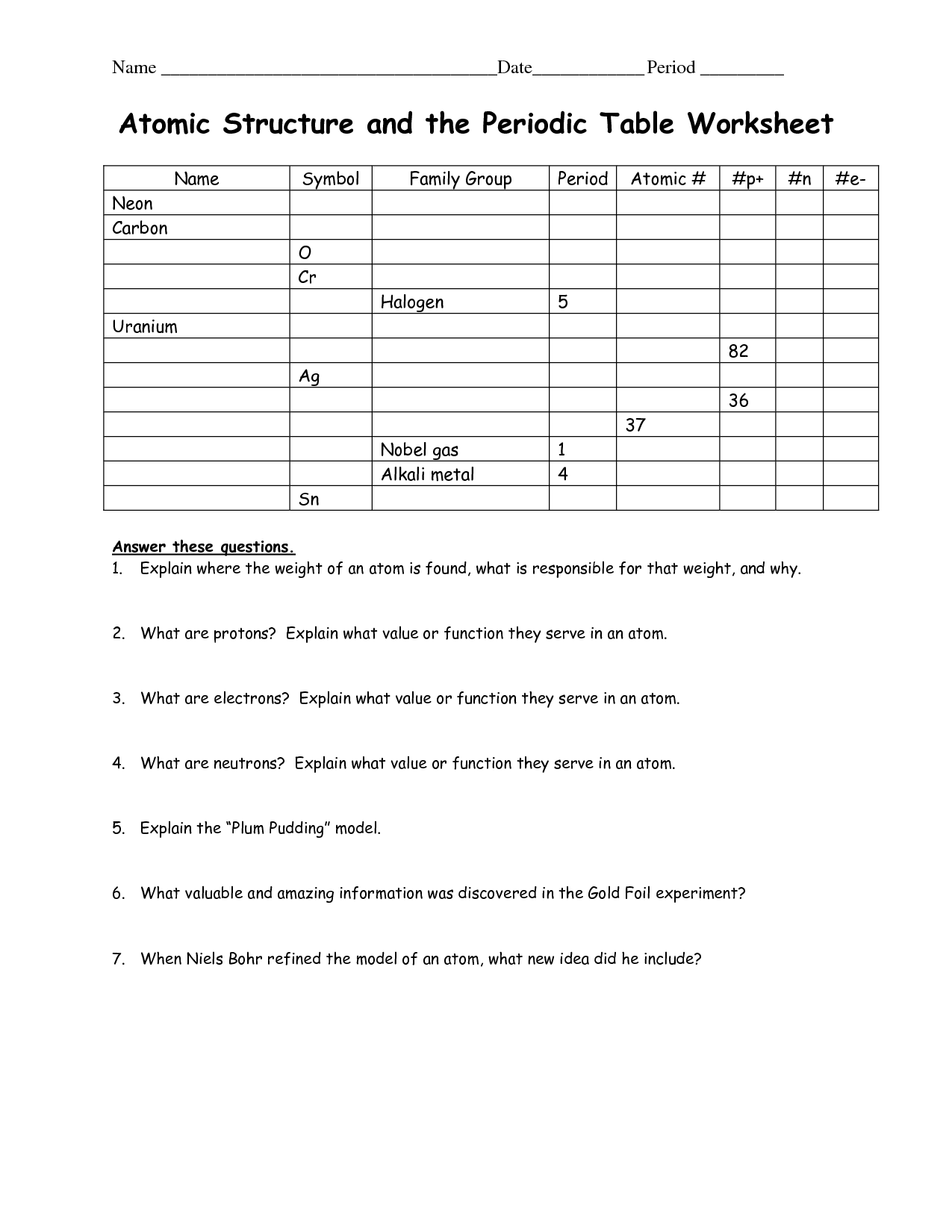
Atomic Structure Worksheet Answers

Understanding the Atomic Structure
Atoms are the basic building blocks of matter, and understanding their structure is crucial in chemistry and physics. The atomic structure consists of three main parts: protons, neutrons, and electrons. In this post, we will delve into the details of the atomic structure and provide answers to common worksheet questions.
The Atomic Model
The atomic model is a representation of the atom, showing the arrangement of protons, neutrons, and electrons. The most widely accepted model is the Rutherford-Bohr model, which depicts the atom as a small, heavy nucleus surrounded by electrons in energy levels or shells.
Components of the Atomic Structure
The atomic structure consists of:
- Protons: positively charged particles found in the nucleus
- Neutrons: particles with no charge found in the nucleus
- Electrons: negatively charged particles that orbit the nucleus
Atomic Number and Mass Number
The atomic number (Z) is the number of protons in an atom's nucleus, which determines the element of an atom. The mass number (A) is the total number of protons and neutrons in an atom's nucleus.
| Term | Definition |
|---|---|
| Atomic Number (Z) | Number of protons in an atom's nucleus |
| Mass Number (A) | Total number of protons and neutrons in an atom's nucleus |
Worksheet Answers
Here are the answers to common worksheet questions on atomic structure:
Question 1: What is the atomic number of an atom with 5 protons?
Answer: 5
📝 Note: The atomic number is equal to the number of protons in an atom's nucleus.
Question 2: What is the mass number of an atom with 6 protons and 8 neutrons?
Answer: 14
📝 Note: The mass number is the total number of protons and neutrons in an atom's nucleus.
Question 3: What is the electron configuration of a neutral atom with 10 electrons?
Answer: 1s² 2s² 2p⁶
📝 Note: The electron configuration shows the arrangement of electrons in an atom's energy levels or shells.
Conclusion
In conclusion, understanding the atomic structure is crucial in chemistry and physics. The atomic model, atomic number, and mass number are essential concepts in understanding the properties of elements. By practicing worksheet questions, you can reinforce your knowledge of atomic structure and improve your problem-solving skills.
What is the atomic structure?
+The atomic structure consists of protons, neutrons, and electrons, with protons and neutrons found in the nucleus and electrons orbiting the nucleus.
What is the difference between atomic number and mass number?
+The atomic number is the number of protons in an atom’s nucleus, while the mass number is the total number of protons and neutrons in an atom’s nucleus.
What is electron configuration?
+Electron configuration shows the arrangement of electrons in an atom’s energy levels or shells.