Types of Chemical Reactions Worksheet Guide
Understanding the Basics of Chemical Reactions
Chemical reactions are a fundamental concept in chemistry, and understanding the different types of reactions is crucial for any aspiring chemist. In this article, we will explore the various types of chemical reactions, including their definitions, examples, and importance.
What are Chemical Reactions?
Chemical reactions involve the transformation of one or more substances into new substances. This process involves the breaking and forming of chemical bonds between atoms, resulting in the creation of new compounds. Chemical reactions can be categorized into several types, each with its own unique characteristics.
Types of Chemical Reactions
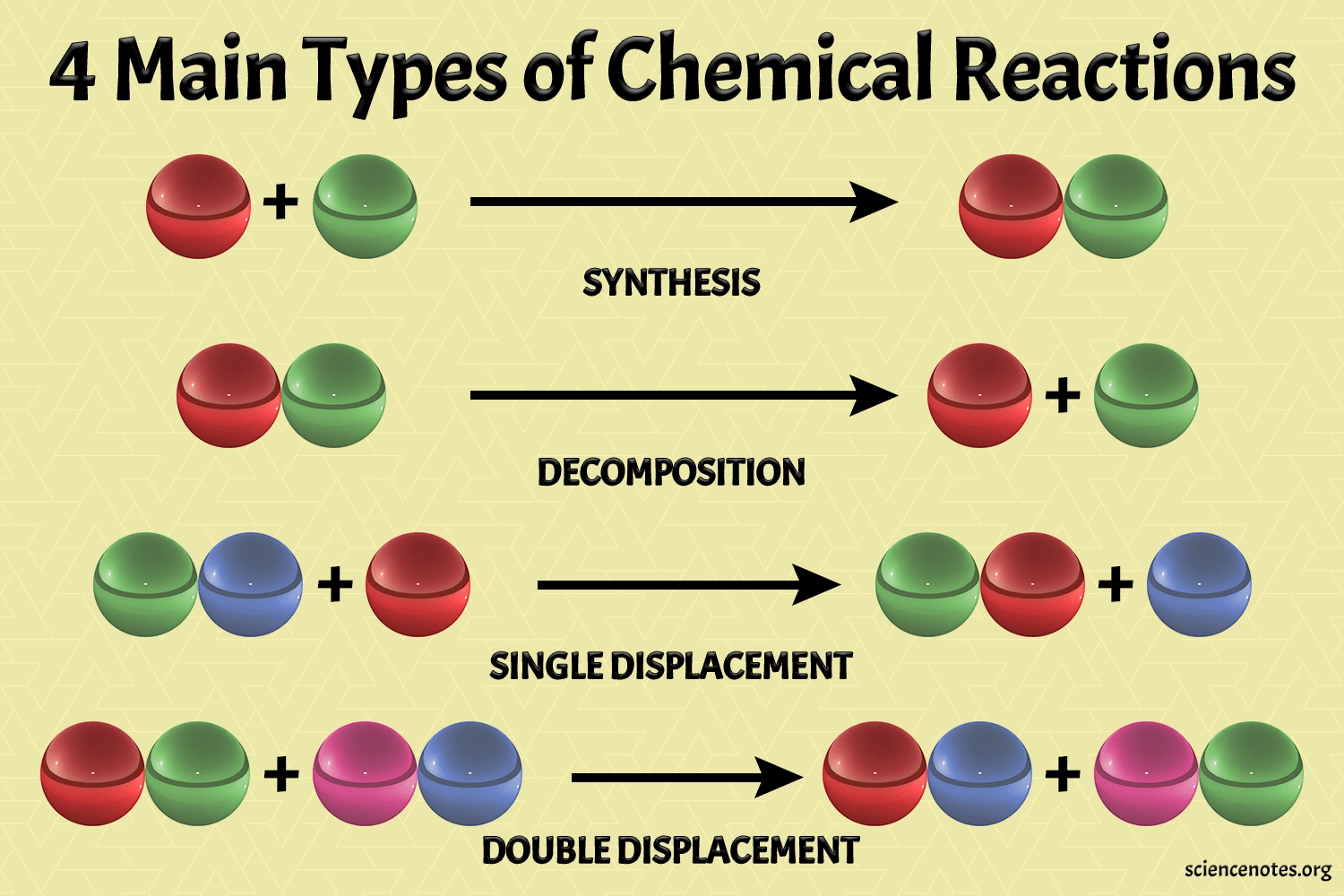
1. Synthesis Reactions
Synthesis reactions involve the combination of two or more substances to form a new compound. This type of reaction is also known as a direct combination reaction.
- Example: 2H2 (g) + O2 (g) → 2H2O (l)
- Importance: Synthesis reactions are used in various industrial processes, such as the production of ammonia and the manufacture of plastics.
2. Decomposition Reactions
Decomposition reactions involve the breakdown of a single compound into two or more simpler substances.
- Example: 2H2O (l) → 2H2 (g) + O2 (g)
- Importance: Decomposition reactions are used in various applications, such as the production of oxygen gas and the decomposition of organic matter.
3. Single Displacement Reactions
Single displacement reactions involve the replacement of one element by another element in a compound.
- Example: Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
- Importance: Single displacement reactions are used in various industrial processes, such as the production of copper and the manufacture of electrical components.
4. Double Displacement Reactions
Double displacement reactions involve the exchange of partners between two compounds.
- Example: NaCl (aq) + AgNO3 (aq) → NaNO3 (aq) + AgCl (s)
- Importance: Double displacement reactions are used in various applications, such as the production of silver chloride and the manufacture of photographic film.
5. Combustion Reactions
Combustion reactions involve the reaction of a substance with oxygen, resulting in the release of heat and light.
- Example: CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l)
- Importance: Combustion reactions are used in various industrial processes, such as the production of energy and the manufacture of chemicals.
6. Neutralization Reactions
Neutralization reactions involve the reaction of an acid with a base, resulting in the formation of a salt and water.
- Example: HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
- Importance: Neutralization reactions are used in various applications, such as the production of soap and the manufacture of pharmaceuticals.
Importance of Understanding Chemical Reactions
Understanding the different types of chemical reactions is crucial for various industrial, environmental, and biological applications. By recognizing the characteristics of each reaction type, scientists and engineers can design and optimize processes to produce desired products, minimize waste, and ensure safety.
👍 Note: Understanding chemical reactions is a fundamental skill for any aspiring chemist, and mastering this concept can lead to exciting career opportunities in fields such as materials science, environmental engineering, and pharmaceutical development.
Conclusion
In conclusion, chemical reactions are a fundamental concept in chemistry, and understanding the different types of reactions is crucial for various industrial, environmental, and biological applications. By recognizing the characteristics of each reaction type, scientists and engineers can design and optimize processes to produce desired products, minimize waste, and ensure safety. Whether you’re a student, researcher, or industry professional, mastering chemical reactions can lead to exciting opportunities and discoveries.
What is the main difference between synthesis and decomposition reactions?
+Synthesis reactions involve the combination of two or more substances to form a new compound, while decomposition reactions involve the breakdown of a single compound into two or more simpler substances.
What is an example of a single displacement reaction?
+Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
Why is understanding chemical reactions important?
+Understanding chemical reactions is crucial for various industrial, environmental, and biological applications, and can lead to exciting career opportunities in fields such as materials science, environmental engineering, and pharmaceutical development.