Mastering Moles and Volume with Practice Worksheets
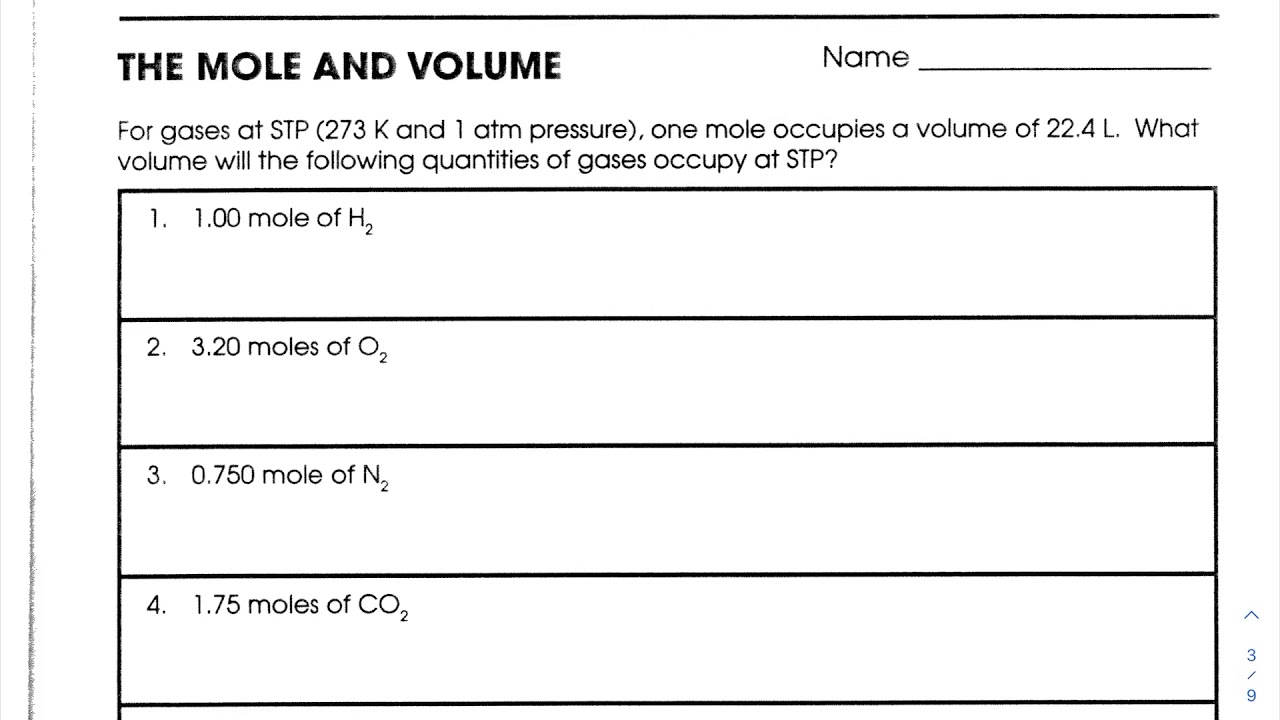
Understanding Moles and Volume
In chemistry, the mole (mol) is a fundamental unit of measurement that represents 6.022 x 10^23 particles (atoms or molecules). It is a crucial concept in understanding chemical reactions, stoichiometry, and quantitative analysis. When combined with volume, moles play a significant role in calculating concentrations, molarities, and volumes of solutions. In this article, we will explore the concept of moles and volume, discuss the relationship between them, and provide practice worksheets to help you master these concepts.
The Mole Concept
The mole is a dimensionless unit that allows us to express the amount of a substance in a convenient and consistent way. It is defined as the amount of a substance that contains as many particles (atoms or molecules) as there are atoms in 0.012 kilograms of carbon-12. This number is known as Avogadro’s number (6.022 x 10^23).
Key Takeaways:
- A mole represents 6.022 x 10^23 particles.
- Moles are used to express the amount of a substance.
- Moles are a dimensionless unit.
Volume and Its Units
Volume is a measure of the amount of space occupied by a substance. In chemistry, volume is typically measured in liters (L) or milliliters (mL). Other units of volume include cubic centimeters (cm^3), cubic meters (m^3), and gallons.
Key Takeaways:
- Volume is a measure of the amount of space occupied by a substance.
- Common units of volume include liters (L), milliliters (mL), cubic centimeters (cm^3), and cubic meters (m^3).
Relationship Between Moles and Volume
The relationship between moles and volume is fundamental in chemistry. When we know the number of moles of a substance and its volume, we can calculate the concentration, molarity, or density of the solution.
Key Takeaways:
- Moles and volume are related through the concept of concentration.
- Knowing the number of moles and volume allows us to calculate molarity, density, and concentration.
Calculating Moles from Volume
To calculate moles from volume, we use the following formula:
moles = volume (L) x molarity (M)
where molarity (M) is the number of moles of solute per liter of solution.
Example 1: Calculate the number of moles of sodium chloride (NaCl) in 2.5 liters of a 0.5 M solution.
moles = 2.5 L x 0.5 M = 1.25 mol
Calculating Volume from Moles
To calculate volume from moles, we use the following formula:
volume (L) = moles / molarity (M)
Example 2: Calculate the volume of a 0.25 M solution of glucose (C6H12O6) that contains 0.75 moles.
volume (L) = 0.75 mol / 0.25 M = 3 L
Practice Worksheets
Practice is key to mastering moles and volume. Here are five practice worksheets to help you reinforce your understanding:
Worksheet 1: Calculate the number of moles of each substance in the given volumes and concentrations.

| Substance | Volume (L) | Concentration (M) | Moles |
|---|---|---|---|
| NaCl | 1.2 L | 0.3 M | ? |
| HCl | 0.8 L | 0.2 M | ? |
| CaCO3 | 3.5 L | 0.1 M | ? |
Worksheet 2: Calculate the volume of each solution given the number of moles and concentration.
| Substance | Moles | Concentration (M) | Volume (L) |
|---|---|---|---|
| KNO3 | 0.5 mol | 0.2 M | ? |
| NaOH | 0.8 mol | 0.4 M | ? |
| H2SO4 | 1.2 mol | 0.6 M | ? |
Worksheet 3: Calculate the molarity of each solution given the number of moles and volume.
| Substance | Moles | Volume (L) | Molarity (M) |
|---|---|---|---|
| NaCl | 0.75 mol | 2 L | ? |
| CaCl2 | 0.4 mol | 1.5 L | ? |
| KI | 0.2 mol | 0.5 L | ? |
Worksheet 4: Calculate the number of moles of each substance in the given masses and molar masses.
| Substance | Mass (g) | Molar Mass (g/mol) | Moles |
|---|---|---|---|
| CO2 | 25 g | 44 g/mol | ? |
| H2O | 18 g | 18 g/mol | ? |
| NaCl | 50 g | 58.5 g/mol | ? |
Worksheet 5: Calculate the volume of each solution given the number of moles and molar mass.
| Substance | Moles | Molar Mass (g/mol) | Volume (L) |
|---|---|---|---|
| H2SO4 | 0.8 mol | 98 g/mol | ? |
| KNO3 | 0.5 mol | 101 g/mol | ? |
| CaCO3 | 0.2 mol | 100 g/mol | ? |
Notes:
📝 Note: Make sure to check your units and significant figures when solving problems.
📝 Note: Use the correct formulas and equations when solving problems.
In conclusion, mastering moles and volume is essential in chemistry. With practice and patience, you can become proficient in calculating moles from volume, volume from moles, and molarity from moles and volume. Remember to check your units and significant figures, and use the correct formulas and equations when solving problems.
What is the definition of a mole?
+A mole is a dimensionless unit that represents 6.022 x 10^23 particles (atoms or molecules).
What is the relationship between moles and volume?
+Moles and volume are related through the concept of concentration. Knowing the number of moles and volume allows us to calculate molarity, density, and concentration.
How do I calculate moles from volume?
+Use the formula: moles = volume (L) x molarity (M)
How do I calculate volume from moles?
+Use the formula: volume (L) = moles / molarity (M)
What are some common units of volume?
+Common units of volume include liters (L), milliliters (mL), cubic centimeters (cm^3), and cubic meters (m^3).