7 Ways to Master Avogadro's Number and Moles
Understanding Avogadro’s number and moles is crucial for any student of chemistry. Mastering these concepts can make a huge difference in your ability to solve problems and think critically about chemical reactions. In this article, we will explore 7 ways to master Avogadro’s number and moles, and provide you with the tools you need to succeed in chemistry.
What is Avogadro's Number?
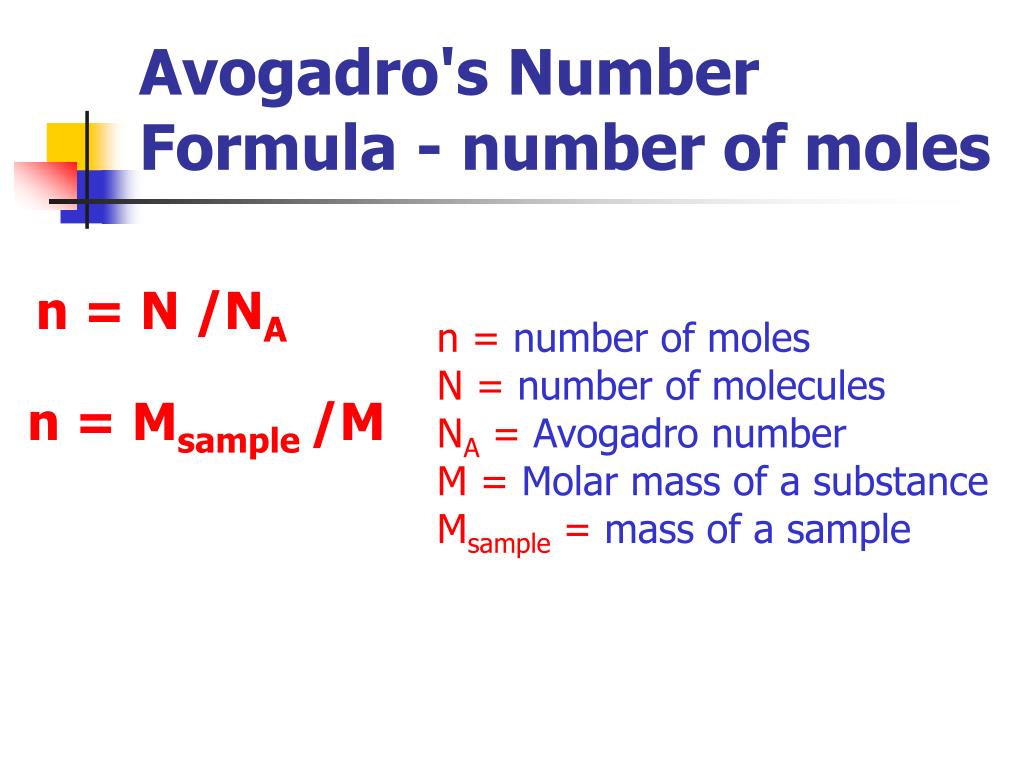
Avogadro’s number is a fundamental constant in chemistry that represents the number of particles (atoms or molecules) in one mole of a substance. It is defined as 6.022 x 10^23 particles per mole. This number is named after the Italian scientist Amedeo Avogadro, who first proposed the idea that equal volumes of gases at the same temperature and pressure contain an equal number of molecules.
What is a Mole?
A mole is a unit of measurement in chemistry that represents 6.022 x 10^23 particles of a substance. It is a way of expressing the amount of a substance in a more convenient and meaningful way than simply using grams or liters. Moles are often used to express the amount of a substance in a chemical reaction or to calculate the number of particles in a given volume of a substance.
7 Ways to Master Avogadro's Number and Moles
1. Understand the Relationship Between Avogadro’s Number and Moles
The key to mastering Avogadro’s number and moles is to understand the relationship between them. Avogadro’s number is the number of particles in one mole of a substance, so if you know the number of moles of a substance, you can calculate the number of particles it contains. Conversely, if you know the number of particles, you can calculate the number of moles.
2. Practice Converting Between Moles and Particles
To master Avogadro’s number and moles, you need to practice converting between moles and particles. Try converting a given number of moles to particles, and vice versa. This will help you to develop a deeper understanding of the relationship between Avogadro’s number and moles.
3. Use Avogadro’s Number to Calculate the Number of Particles in a Substance
Avogadro’s number can be used to calculate the number of particles in a substance. If you know the number of moles of a substance, you can multiply it by Avogadro’s number to get the number of particles. For example, if you have 2 moles of carbon dioxide, you can calculate the number of particles as follows:
2 moles x 6.022 x 10^23 particles/mole = 1.2044 x 10^24 particles
4. Use Moles to Calculate the Amount of a Substance in a Chemical Reaction
Moles can be used to calculate the amount of a substance in a chemical reaction. If you know the number of moles of a substance, you can calculate the amount of the substance in grams or liters. For example, if you have 2 moles of carbon dioxide, you can calculate the amount of carbon dioxide in grams as follows:
2 moles x 44 g/mole = 88 g
5. Understand the Concept of Molar Mass
Molar mass is the mass of one mole of a substance. It is a way of expressing the mass of a substance in a more convenient and meaningful way than simply using grams. Molar mass can be used to calculate the amount of a substance in a chemical reaction.
6. Practice Calculating Molar Mass
To master Avogadro’s number and moles, you need to practice calculating molar mass. Try calculating the molar mass of different substances, and use it to calculate the amount of the substance in a chemical reaction.
7. Use Online Resources to Practice and Reinforce Your Understanding
There are many online resources available to help you practice and reinforce your understanding of Avogadro’s number and moles. Try using online calculators or worksheets to practice converting between moles and particles, and to calculate molar mass.
📝 Note: Practice is key to mastering Avogadro's number and moles. Try to practice regularly, and use online resources to reinforce your understanding.
Conclusion
Mastering Avogadro’s number and moles is crucial for any student of chemistry. By following these 7 ways, you can develop a deeper understanding of the relationship between Avogadro’s number and moles, and improve your ability to solve problems and think critically about chemical reactions. Remember to practice regularly, and use online resources to reinforce your understanding.
What is Avogadro’s number?
+Avogadro’s number is a fundamental constant in chemistry that represents the number of particles (atoms or molecules) in one mole of a substance. It is defined as 6.022 x 10^23 particles per mole.
What is a mole?
+A mole is a unit of measurement in chemistry that represents 6.022 x 10^23 particles of a substance. It is a way of expressing the amount of a substance in a more convenient and meaningful way than simply using grams or liters.
How do I calculate the number of particles in a substance?
+You can calculate the number of particles in a substance by multiplying the number of moles by Avogadro’s number. For example, if you have 2 moles of carbon dioxide, you can calculate the number of particles as follows: 2 moles x 6.022 x 10^23 particles/mole = 1.2044 x 10^24 particles.