5 Easy Steps to Master Density Calculations
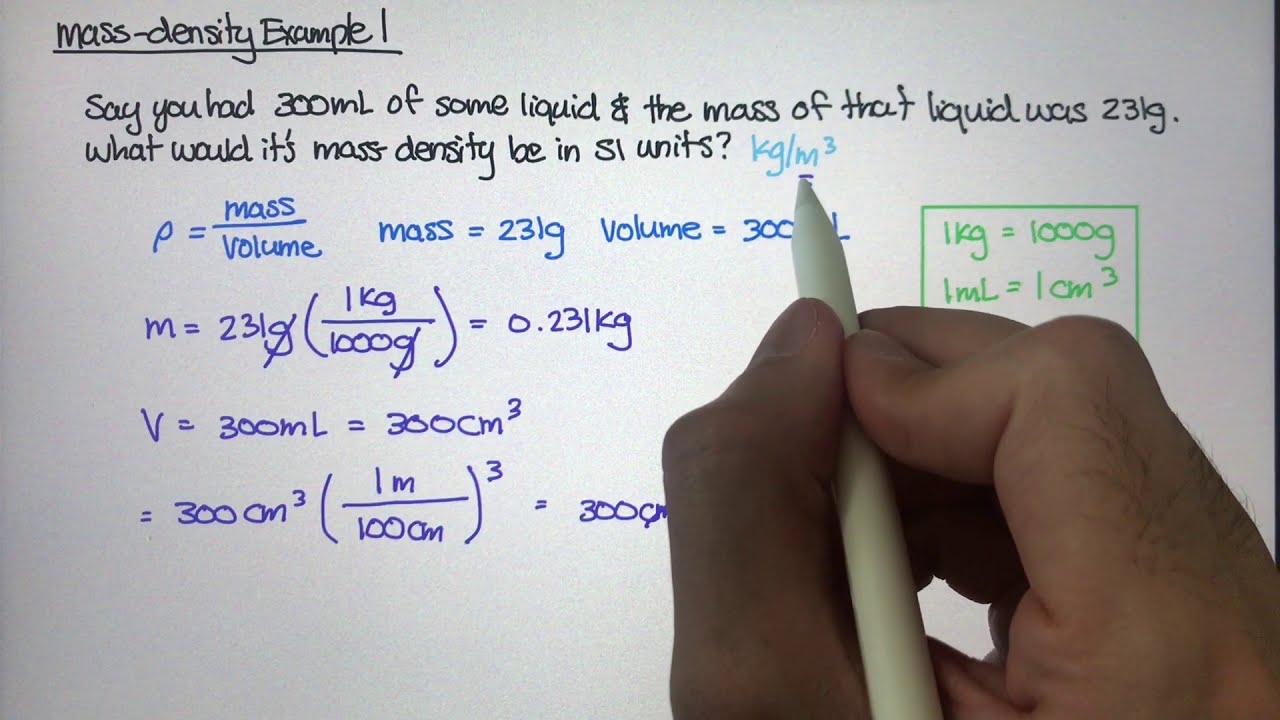
Understanding Density and Its Importance
Density is a fundamental concept in physics and engineering that describes the amount of mass contained in a given unit volume of a substance. It is a crucial property that helps us understand the behavior of materials under different conditions. Density calculations are used in various fields, including chemistry, physics, engineering, and materials science. In this article, we will guide you through 5 easy steps to master density calculations.
Step 1: Understand the Formula for Density
The formula for density is simple and straightforward:
density = mass / volume
where density is typically denoted by the symbol ρ (rho), mass is denoted by m, and volume is denoted by V.
📝 Note: Make sure to use the correct units for mass (e.g., kilograms, grams) and volume (e.g., cubic meters, liters).
Step 2: Identify the Units of Measurement
Before performing density calculations, it is essential to identify the units of measurement for mass and volume. Common units for mass include:
- Kilograms (kg)
- Grams (g)
- Milligrams (mg)
Common units for volume include:
- Cubic meters (m³)
- Liters (L)
- Milliliters (mL)
Step 3: Convert Units if Necessary
If the units of measurement for mass and volume are not compatible, you may need to convert them before performing the calculation. For example, if the mass is given in grams and the volume is given in liters, you can convert the mass to kilograms or the volume to cubic meters.
Here’s a simple conversion table:

| Unit | Conversion Factor |
|---|---|
| 1 kg | 1000 g |
| 1 L | 0.001 m³ |
| 1 mL | 0.000001 m³ |
Step 4: Plug in the Values and Calculate
Once you have identified the units of measurement and converted them if necessary, you can plug in the values into the formula:
density = mass / volume
For example, if the mass is 50 grams and the volume is 100 milliliters, the calculation would be:
density = 50 g / 100 mL
To convert the units, we can use the conversion factors:
density = 0.05 kg / 0.0001 m³
density = 500 kg/m³
Step 5: Interpret the Results
After calculating the density, it is essential to interpret the results. Density can be used to:
- Identify the substance (e.g., density of water is approximately 1000 kg/m³)
- Determine the amount of mass contained in a given volume
- Calculate the volume of a substance given its mass and density
In conclusion, mastering density calculations requires a basic understanding of the formula, units of measurement, and conversion factors. By following these 5 easy steps, you can become proficient in calculating density and apply it to various problems in physics, engineering, and materials science.
What is the formula for density?
+The formula for density is: density = mass / volume
What are the common units of measurement for mass and volume?
+Common units for mass include kilograms, grams, and milligrams. Common units for volume include cubic meters, liters, and milliliters.
Why is it essential to convert units before performing density calculations?
+Converting units ensures that the units of measurement for mass and volume are compatible, which is essential for accurate calculations.