Quantum Numbers Worksheet

Understanding Quantum Numbers: A Fundamental Concept in Chemistry
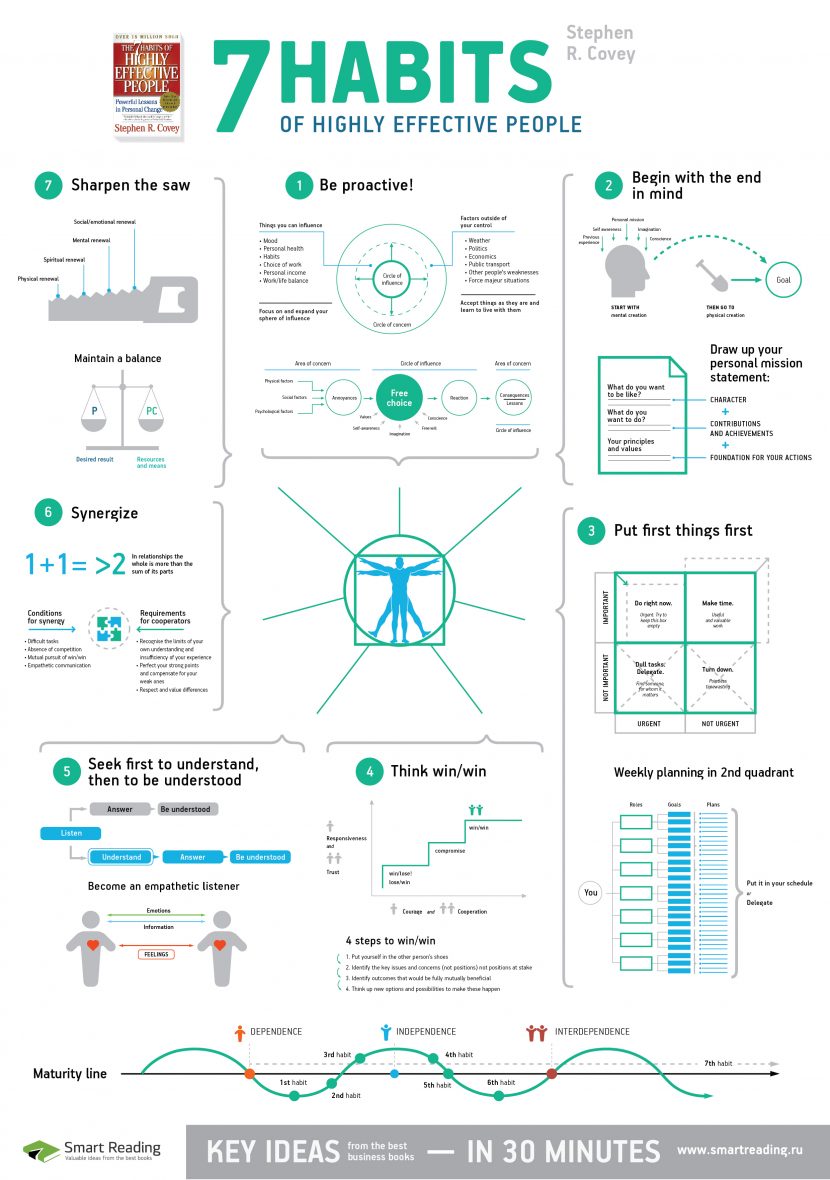
In the realm of chemistry, quantum numbers play a crucial role in describing the behavior of electrons within an atom. These numbers provide a unique set of values that define the energy, shape, and orientation of an electron’s orbital. In this blog post, we will delve into the world of quantum numbers, exploring their significance, types, and applications.
The Significance of Quantum Numbers
Quantum numbers are a set of four numbers that describe the energy, shape, and orientation of an electron’s orbital in an atom. These numbers are essential in understanding the electronic configuration of an atom, which in turn helps us predict the chemical properties of an element. The four quantum numbers are:
- Principal Quantum Number (n): describes the energy level of the electron
- Azimuthal Quantum Number (l): describes the shape of the orbital
- Magnetic Quantum Number (m): describes the orientation of the orbital
- Spin Quantum Number (s): describes the spin of the electron
Types of Quantum Numbers
Each type of quantum number has its unique set of values, which are essential in defining the electronic configuration of an atom.
- Principal Quantum Number (n): can take any positive integer value (1, 2, 3,…), describing the energy level of the electron
- Azimuthal Quantum Number (l): can take values from 0 to n-1, describing the shape of the orbital
- Magnetic Quantum Number (m): can take values from -l to +l, describing the orientation of the orbital
- Spin Quantum Number (s): can take values of +1⁄2 or -1⁄2, describing the spin of the electron
Applications of Quantum Numbers
Quantum numbers have numerous applications in chemistry and physics, including:
- Electronic Configuration: quantum numbers help us predict the electronic configuration of an atom, which in turn helps us understand the chemical properties of an element
- Periodic Table: quantum numbers are used to explain the periodic trends in the periodic table, such as the atomic radius and electronegativity
- Chemical Bonding: quantum numbers help us understand the formation of chemical bonds and the molecular structure of compounds
- Spectroscopy: quantum numbers are used to interpret the spectra of atoms and molecules
Quantum Numbers Worksheet
Here is a worksheet to help you practice your understanding of quantum numbers:
| Atom | Principal Quantum Number (n) | Azimuthal Quantum Number (l) | Magnetic Quantum Number (m) | Spin Quantum Number (s) |
|---|---|---|---|---|
| Hydrogen | 1 | 0 | 0 | +1⁄2 |
| Helium | 1 | 0 | 0 | -1⁄2 |
| Lithium | 2 | 0 | 0 | +1⁄2 |
| Beryllium | 2 | 1 | -1 | +1⁄2 |
| Boron | 2 | 1 | 0 | +1⁄2 |
Complete the table by filling in the missing values.
Solutions to the Worksheet
Here are the solutions to the worksheet:
| Atom | Principal Quantum Number (n) | Azimuthal Quantum Number (l) | Magnetic Quantum Number (m) | Spin Quantum Number (s) |
|---|---|---|---|---|
| Hydrogen | 1 | 0 | 0 | +1⁄2 |
| Helium | 1 | 0 | 0 | -1⁄2 |
| Lithium | 2 | 0 | 0 | +1⁄2 |
| Beryllium | 2 | 1 | -1 | +1⁄2 |
| Boron | 2 | 1 | 0 | +1⁄2 |
| Carbon | 2 | 1 | +1 | +1⁄2 |
| Nitrogen | 2 | 1 | +1 | -1⁄2 |
| Oxygen | 2 | 2 | -2 | +1⁄2 |
📝 Note: The values in the table are for the outermost energy level of each atom.
Conclusion
In conclusion, quantum numbers are a fundamental concept in chemistry that helps us understand the electronic configuration of an atom. By mastering the four types of quantum numbers, we can predict the chemical properties of an element and understand the periodic trends in the periodic table. The worksheet provided above is a great way to practice your understanding of quantum numbers, and the solutions can be used as a reference.
What are the four types of quantum numbers?
+The four types of quantum numbers are: Principal Quantum Number (n), Azimuthal Quantum Number (l), Magnetic Quantum Number (m), and Spin Quantum Number (s).
What is the significance of quantum numbers in chemistry?
+Quantum numbers help us understand the electronic configuration of an atom, which in turn helps us predict the chemical properties of an element.
What is the principal quantum number (n) used for?
+The principal quantum number (n) is used to describe the energy level of the electron.