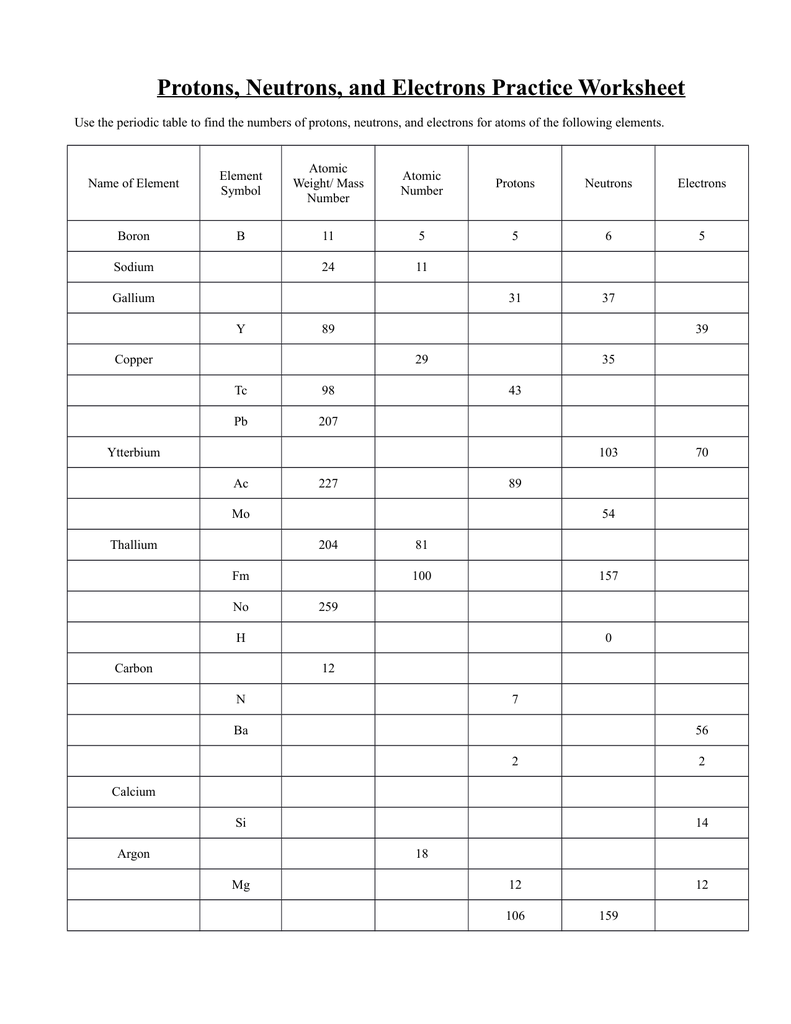
Protons Neutrons Electrons Worksheet Answer Key
Understanding the Building Blocks of Matter: Protons, Neutrons, and Electrons
Atoms are the fundamental units of matter, and they consist of three main components: protons, neutrons, and electrons. Understanding the roles and relationships between these particles is crucial for grasping various concepts in chemistry and physics. This article will delve into the basics of protons, neutrons, and electrons, followed by a comprehensive worksheet answer key to help reinforce your understanding.
Protons
Protons are positively charged particles that reside in the nucleus of an atom. The number of protons in an atom determines the element of an atom, and each element has a unique number of protons in its atoms. This number is known as the atomic number.
Neutrons
Neutrons have no charge and are found in the nucleus along with protons. The number of neutrons in an atom can vary, leading to different isotopes of the same element. Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons.
Electrons
Electrons are negatively charged particles that orbit the nucleus of an atom. The number of electrons in a neutral atom is equal to the number of protons, and this balance is crucial for the atom’s stability. Electrons are arranged in energy levels or electron shells around the nucleus.
Worksheet Answer Key
Section 1: Multiple Choice Questions
Which particle is positively charged?
- A) Electron
- B) Proton
- C) Neutron
- Answer: B) Proton
What determines the element of an atom?
- A) Number of electrons
- B) Number of neutrons
- C) Number of protons
- Answer: C) Number of protons
What are atoms of the same element with different numbers of neutrons called?
- A) Isotopes
- B) Elements
- C) Compounds
- Answer: A) Isotopes
Section 2: Short Answer Questions
Describe the relationship between protons and electrons in a neutral atom.
- Answer: In a neutral atom, the number of protons is equal to the number of electrons. This balance is necessary for the atom’s stability.
What is the primary difference between isotopes of the same element?
- Answer: Isotopes of the same element have the same number of protons but differ in the number of neutrons.
Section 3: Fill in the Blanks
The nucleus of an atom contains _______________________ and _______________________.
- Answer: protons and neutrons
Electrons are arranged in _______________________ around the nucleus.
- Answer: energy levels or electron shells
Section 4: True or False
- All atoms of the same element have the same number of neutrons. (False: Only atoms of the same isotope have the same number of neutrons.)
- Protons have a negative charge. (False: Protons have a positive charge.)
- Neutrons are found in the electron cloud. (False: Neutrons are found in the nucleus.)
Conclusion
Understanding the roles and properties of protons, neutrons, and electrons is fundamental to understanding chemistry and physics. The balance between protons and electrons in atoms and the variation in neutron numbers leading to isotopes are crucial concepts. Using the worksheet answer key provided, you can assess your understanding and reinforce your knowledge of these atomic components.
What is the charge of a neutron?
+Neutrons have no charge.
Why is the number of protons important in an atom?
+The number of protons determines the element of an atom.
Can atoms of the same element have different numbers of electrons?
+No, atoms of the same element have the same number of electrons in a neutral state.