5 Essential Polyatomic Ion Worksheet Answers
Mastering Polyatomic Ions: A Comprehensive Guide with Worksheet Answers
Polyatomic ions are a crucial concept in chemistry, and understanding them is essential for mastering various chemical reactions and formulas. In this article, we will delve into the world of polyatomic ions, exploring their definition, types, and significance. Additionally, we will provide answers to 5 essential polyatomic ion worksheet questions to help you reinforce your knowledge.
What are Polyatomic Ions?
Polyatomic ions, also known as molecular ions or complex ions, are ions that consist of two or more atoms covalently bonded together. They can be either positively or negatively charged, depending on the number of electrons they have gained or lost. Polyatomic ions are commonly found in ionic compounds, where they are combined with other ions to form a neutral compound.
Types of Polyatomic Ions
There are several types of polyatomic ions, including:
- Nitrate (NO3-): A negatively charged ion composed of one nitrogen atom and three oxygen atoms.
- Sulfate (SO42-): A negatively charged ion composed of one sulfur atom and four oxygen atoms.
- Phosphate (PO43-): A negatively charged ion composed of one phosphorus atom and four oxygen atoms.
- Ammonium (NH4+): A positively charged ion composed of one nitrogen atom and four hydrogen atoms.
- Carbonate (CO32-): A negatively charged ion composed of one carbon atom and three oxygen atoms.
Significance of Polyatomic Ions
Polyatomic ions play a vital role in various chemical reactions and processes. They are essential for:
- Forming ionic compounds: Polyatomic ions combine with other ions to form ionic compounds, which are commonly found in nature.
- Acid-base reactions: Polyatomic ions can act as acids or bases, influencing the pH of a solution.
- Electrolysis: Polyatomic ions can participate in electrolysis reactions, where they are reduced or oxidized.
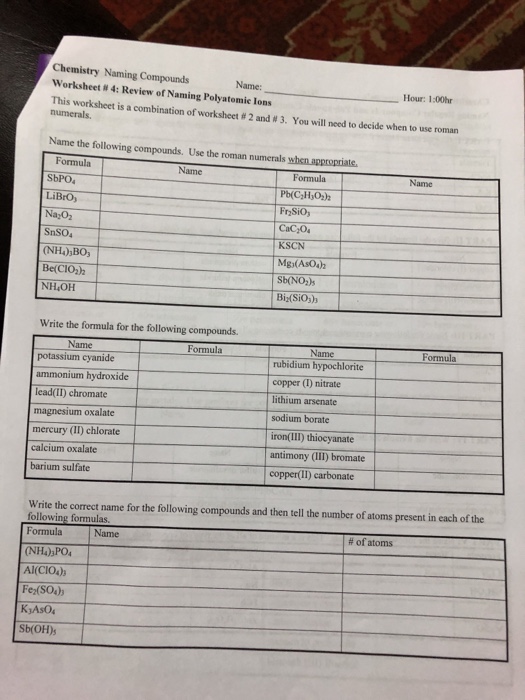
5 Essential Polyatomic Ion Worksheet Answers
Here are the answers to 5 essential polyatomic ion worksheet questions:
Question 1: What is the formula for the nitrate ion?
Answer: NO3-
Question 2: Which polyatomic ion is composed of one sulfur atom and four oxygen atoms?
Answer: Sulfate (SO42-)
Question 3: What is the charge of the phosphate ion?
Answer: -3
Question 4: Which polyatomic ion is composed of one nitrogen atom and four hydrogen atoms?
Answer: Ammonium (NH4+)
Question 5: What is the formula for the carbonate ion?
Answer: CO32-
[💡] Note: Make sure to memorize the formulas and charges of common polyatomic ions to excel in chemistry!
Conclusion
Polyatomic ions are a fundamental concept in chemistry, and understanding their definition, types, and significance is crucial for mastering various chemical reactions and formulas. By practicing with worksheet questions and answers, you can reinforce your knowledge and become more confident in your ability to work with polyatomic ions.
What is the difference between a polyatomic ion and a molecular compound?
+A polyatomic ion is a charged particle composed of two or more atoms covalently bonded together, whereas a molecular compound is a neutral compound composed of two or more atoms covalently bonded together.
How do I determine the charge of a polyatomic ion?
+The charge of a polyatomic ion can be determined by counting the number of electrons gained or lost by the ion. A polyatomic ion with a negative charge has gained electrons, while a polyatomic ion with a positive charge has lost electrons.
What is the significance of polyatomic ions in acid-base reactions?
+Polyatomic ions can act as acids or bases, influencing the pH of a solution. For example, the sulfate ion (SO42-) can accept a proton (H+) to form sulfuric acid (H2SO4), making it an acid.