5 Steps to Master Naming Binary Compounds Covalent

Mastering the Art of Naming Binary Compounds Covalent
Naming binary compounds covalent can seem daunting at first, but with practice and a clear understanding of the rules, you’ll become a pro in no time. In this article, we’ll break down the process into five manageable steps. By the end of this article, you’ll be able to confidently name any binary compound covalent that comes your way.
Step 1: Identify the Type of Compound
Before we dive into naming, it’s essential to identify the type of compound you’re dealing with. Binary compounds covalent are composed of two nonmetal elements. These elements can be from the same group (e.g., oxygen and sulfur) or different groups (e.g., carbon and oxygen).
💡 Note: Make sure to check the periodic table to determine the group and classification of each element.
Step 2: Determine the Prefixes
Binary compounds covalent use prefixes to indicate the number of atoms of each element present in the compound. The prefixes are as follows:
- Mono- (1 atom)
- Di- (2 atoms)
- Tri- (3 atoms)
- Tetra- (4 atoms)
- Penta- (5 atoms)
- Hexa- (6 atoms)
- Hepta- (7 atoms)
- Octa- (8 atoms)
- Nona- (9 atoms)
- Deca- (10 atoms)
For example, if a compound contains one carbon atom and four oxygen atoms, the prefix would be “mon-” for carbon and “tetra-” for oxygen.
Step 3: Name the First Element
The first element in the compound is named using its standard name. If the first element is a single atom, the prefix “mon-” is usually omitted. For example, in the compound CO2, the first element is carbon, which is named “carbon.”
Step 4: Name the Second Element
The second element is named by adding the suffix “-ide” to the root of the element’s name. For example, in the compound CO2, the second element is oxygen, which is named “oxide.”
Step 5: Combine the Names
Now it’s time to combine the names of the two elements. The prefix for the second element is added to the root of the element’s name, followed by the suffix “-ide.” The name of the first element is added to the beginning of the compound name.
Using the example from Step 2, the compound CO2 would be named “carbon tetraoxide.”
| Element 1 | Prefix | Element 2 | Prefix | Compound Name |
|---|---|---|---|---|
| Carbon (C) | mon- | Oxygen (O) | tetra- | Carbon tetraoxide |
| Sulfur (S) | di- | Oxygen (O) | pent- | Disulfur pentoxide |
| Nitrogen (N) | tri- | Fluorine (F) | hex- | Trinitrogen hexafluoride |
By following these five steps, you’ll be able to name any binary compound covalent with ease.
In summary, to name binary compounds covalent, you need to identify the type of compound, determine the prefixes, name the first element, name the second element, and combine the names. With practice, you’ll become proficient in naming these compounds, and it will become second nature.
What is the difference between binary compounds covalent and ionic compounds?
+Binary compounds covalent are composed of two nonmetal elements, whereas ionic compounds are composed of a metal and a nonmetal element.
Why are prefixes used in naming binary compounds covalent?
+Prefixes are used to indicate the number of atoms of each element present in the compound.
What is the correct suffix for the second element in a binary compound covalent?
+The suffix “-ide” is added to the root of the element’s name.
Related Terms:
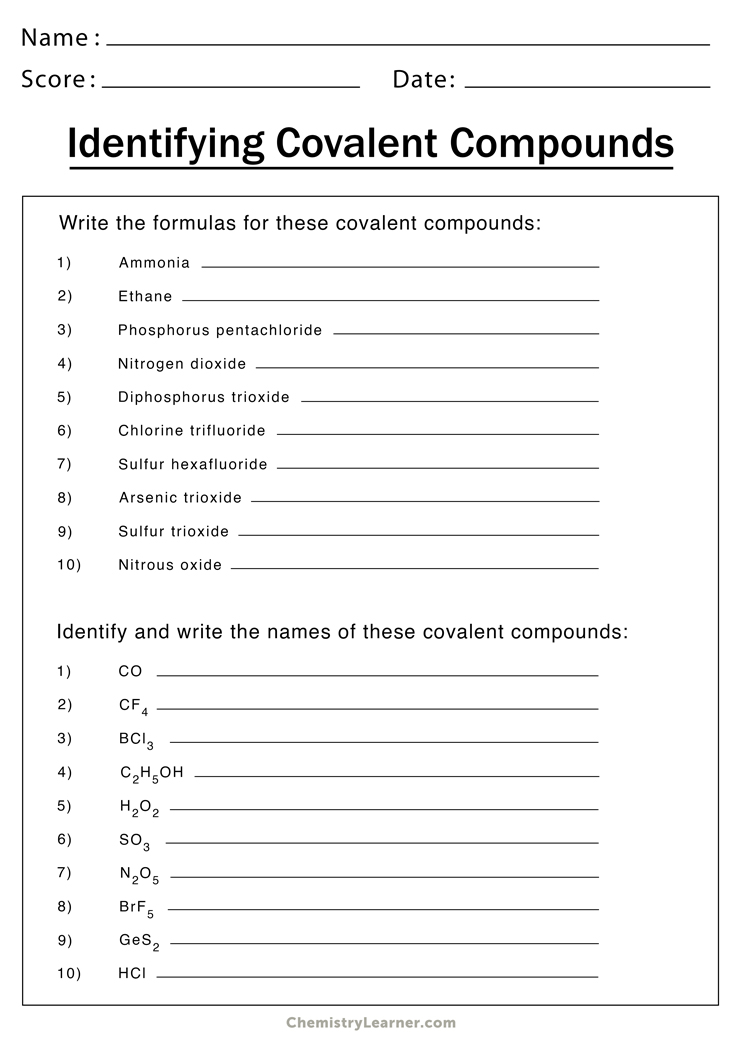
- Naming binary covalent compounds Worksheet
- Naming binary ionic compounds Worksheet
- Naming chemical compounds Worksheet
- Ionic Binary compounds Worksheet