Master Polyatomic Ions with These 10 Essential Tips
Mastering polyatomic ions is a crucial step in understanding chemistry, particularly in the realm of inorganic chemistry. Polyatomic ions are groups of atoms that have a charge, either positive or negative, and they play a significant role in the formation of many compounds. Here are 10 essential tips to help you master polyatomic ions.
1. Understand the Definition and Importance of Polyatomic Ions
Polyatomic ions are groups of two or more atoms that have a charge. They can be either cations (positively charged) or anions (negatively charged). Polyatomic ions are important because they are the building blocks of many compounds, and understanding them is crucial for predicting the properties and behavior of these compounds.
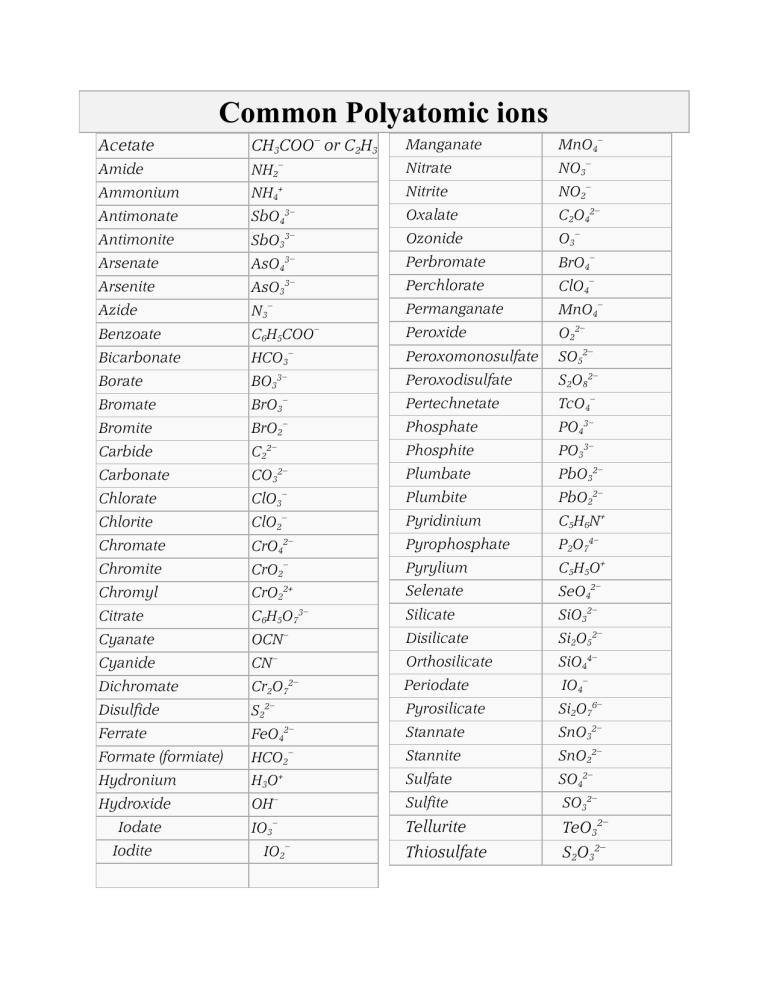
2. Learn the Most Common Polyatomic Ions
There are many polyatomic ions, but some are more common than others. It’s essential to learn the most common ones, such as:
- Ammonium (NH4+)
- Hydroxide (OH-)
- Carbonate (CO32-)
- Bicarbonate (HCO3-)
- Nitrate (NO3-)
- Sulfate (SO42-)
- Phosphate (PO43-)
3. Recognize the Charges of Polyatomic Ions
Each polyatomic ion has a specific charge, and it’s essential to recognize these charges. For example:
- Ammonium (NH4+) has a +1 charge
- Hydroxide (OH-) has a -1 charge
- Carbonate (CO32-) has a -2 charge
4. Understand How Polyatomic Ions Form Compounds
Polyatomic ions form compounds by combining with other ions or groups of ions. For example:
- Sodium hydroxide (NaOH) forms when sodium (Na+) combines with hydroxide (OH-)
- Calcium carbonate (CaCO3) forms when calcium (Ca2+) combines with carbonate (CO32-)
5. Learn the Rules for Writing Formulas with Polyatomic Ions
When writing formulas with polyatomic ions, there are specific rules to follow:
- Write the cation first, followed by the anion
- Use parentheses to group polyatomic ions
- Use subscripts to indicate the number of atoms in a polyatomic ion
For example:
- Sodium hydroxide: NaOH
- Calcium carbonate: CaCO3
- Ammonium phosphate: (NH4)3PO4
6. Practice Writing Formulas with Polyatomic Ions
Practice makes perfect! Write formulas for compounds that contain polyatomic ions. For example:
- Write the formula for potassium nitrate: KNO3
- Write the formula for magnesium sulfate: MgSO4
- Write the formula for ammonium bicarbonate: (NH4)HCO3
7. Learn the Names of Polyatomic Ions
Each polyatomic ion has a specific name, and it’s essential to learn these names. For example:
- Hydroxide: OH-
- Carbonate: CO32-
- Sulfate: SO42-
8. Understand the Relationship Between Polyatomic Ions and Acids
Many polyatomic ions are related to acids. For example:
- Hydroxide (OH-) is related to hydrochloric acid (HCl)
- Carbonate (CO32-) is related to carbonic acid (H2CO3)
- Sulfate (SO42-) is related to sulfuric acid (H2SO4)
9. Use Tables and Charts to Help You Memorize Polyatomic Ions
Tables and charts can be helpful tools for memorizing polyatomic ions. Create a table or chart that lists the most common polyatomic ions, their charges, and their formulas.
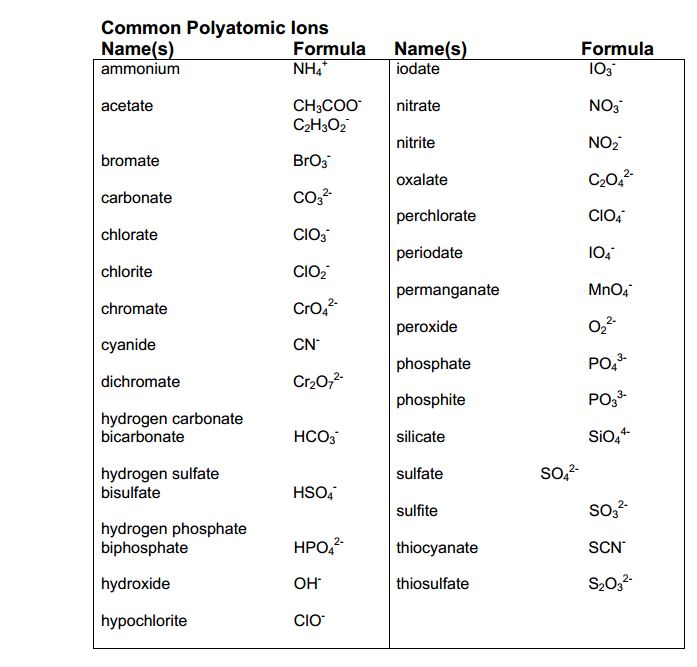
| Polyatomic Ion | Charge | Formula |
|---|---|---|
| Ammonium | +1 | NH4+ |
| Hydroxide | -1 | OH- |
| Carbonate | -2 | CO32- |
10. Practice, Practice, Practice!
The key to mastering polyatomic ions is practice. Practice writing formulas, naming compounds, and identifying the charges of polyatomic ions. The more you practice, the more confident you’ll become.
📝 Note: Mastering polyatomic ions takes time and practice. Be patient, and don't be afraid to make mistakes. With consistent practice, you'll become a pro at working with polyatomic ions!
Mastering polyatomic ions is a crucial step in understanding chemistry, particularly in the realm of inorganic chemistry. By following these 10 essential tips, you’ll be well on your way to becoming a master of polyatomic ions. Remember to practice, practice, practice, and don’t be afraid to make mistakes. With time and effort, you’ll become a pro at working with polyatomic ions.
What is a polyatomic ion?
+A polyatomic ion is a group of two or more atoms that have a charge, either positive or negative.
How do polyatomic ions form compounds?
+Polyatomic ions form compounds by combining with other ions or groups of ions.
What is the most common polyatomic ion?
+The most common polyatomic ions include ammonium (NH4+), hydroxide (OH-), and carbonate (CO32-).