pH Calculations Made Easy with This Worksheet
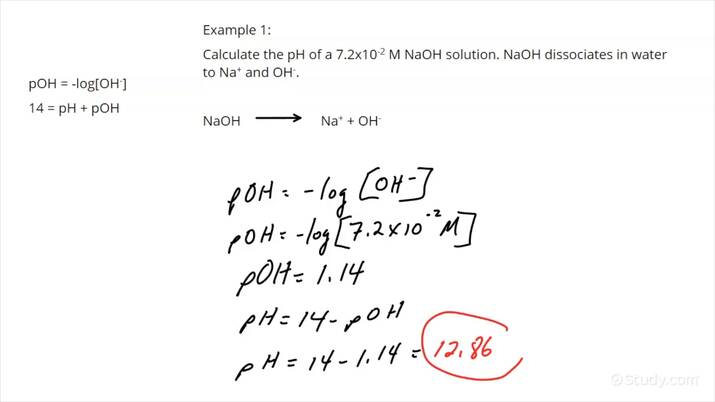
Understanding pH Calculations
pH calculations can be a daunting task for many students and professionals alike. However, with the right tools and a clear understanding of the concepts, it can be made easy. In this article, we will provide a comprehensive worksheet that will guide you through the process of pH calculations, making it a breeze for you.
What is pH?
Before we dive into the calculations, let’s first understand what pH is. pH is a measure of the concentration of hydrogen ions in a solution. It is a scale that ranges from 0 to 14, with a pH of 7 being neutral, below 7 being acidic, and above 7 being basic.
Types of pH Calculations
There are several types of pH calculations, including:
- Strong acid-strong base titrations: These involve the reaction of a strong acid with a strong base.
- Weak acid-strong base titrations: These involve the reaction of a weak acid with a strong base.
- Strong acid-weak base titrations: These involve the reaction of a strong acid with a weak base.
- Weak acid-weak base titrations: These involve the reaction of a weak acid with a weak base.
pH Calculation Worksheet
Here is a comprehensive worksheet that will guide you through the process of pH calculations:
| Type of Titration | Equation | pH Calculation |
|---|---|---|
| Strong acid-strong base | HCl + NaOH → NaCl + H2O | pH = -log[H+] |
| Weak acid-strong base | CH3COOH + NaOH → CH3COONa + H2O | pH = pKa + log([A-]/[HA]) |
| Strong acid-weak base | HCl + NH3 → NH4Cl | pH = pKb + log([BH+]/[B]) |
| Weak acid-weak base | CH3COOH + NH3 → CH3COONH4 | pH = (pKa + pKb)/2 + log([A-]/[HA]) |
📝 Note: The equations and pH calculations above are simplified and assume that the concentrations of the acid and base are equal.
How to Use the Worksheet
To use the worksheet, simply follow these steps:
- Identify the type of titration you are performing.
- Write down the equation for the reaction.
- Use the pH calculation formula corresponding to the type of titration.
- Plug in the values for the concentrations of the acid and base.
- Calculate the pH using a calculator or spreadsheet.
Example Problem
Suppose we are performing a strong acid-strong base titration between HCl and NaOH. The concentration of HCl is 0.1 M and the concentration of NaOH is 0.2 M. What is the pH of the solution after the reaction is complete?
Using the worksheet, we can see that the equation for the reaction is:
HCl + NaOH → NaCl + H2O
The pH calculation formula for strong acid-strong base titrations is:
pH = -log[H+]
Plugging in the values for the concentrations of HCl and NaOH, we get:
pH = -log(0.1) = 1
Therefore, the pH of the solution after the reaction is complete is 1.
Conclusion
pH calculations don’t have to be difficult. With the right tools and a clear understanding of the concepts, you can easily calculate the pH of a solution. The worksheet provided above is a comprehensive guide that will walk you through the process of pH calculations, making it a breeze for you.
What is pH?
+pH is a measure of the concentration of hydrogen ions in a solution.
What is the pH scale?
+The pH scale ranges from 0 to 14, with a pH of 7 being neutral, below 7 being acidic, and above 7 being basic.
What is the difference between a strong acid and a weak acid?
+A strong acid is an acid that completely dissociates in water, while a weak acid is an acid that only partially dissociates in water.