Periodic Trends Worksheet 1 Answer Key
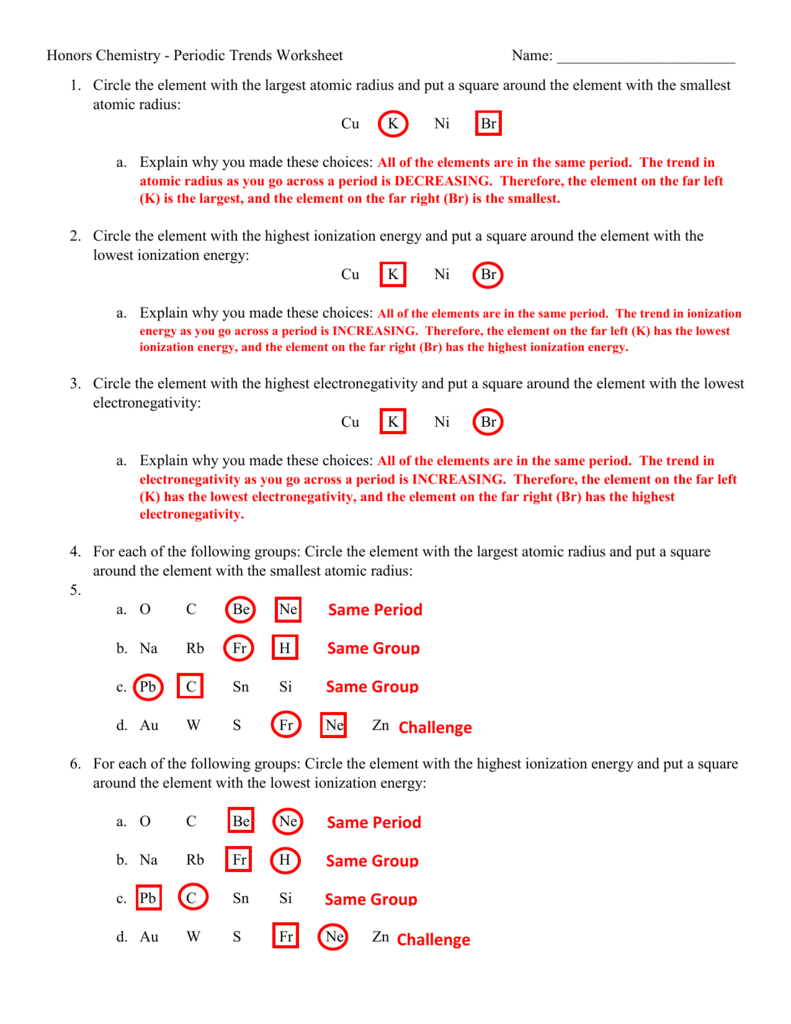
Understanding Periodic Trends
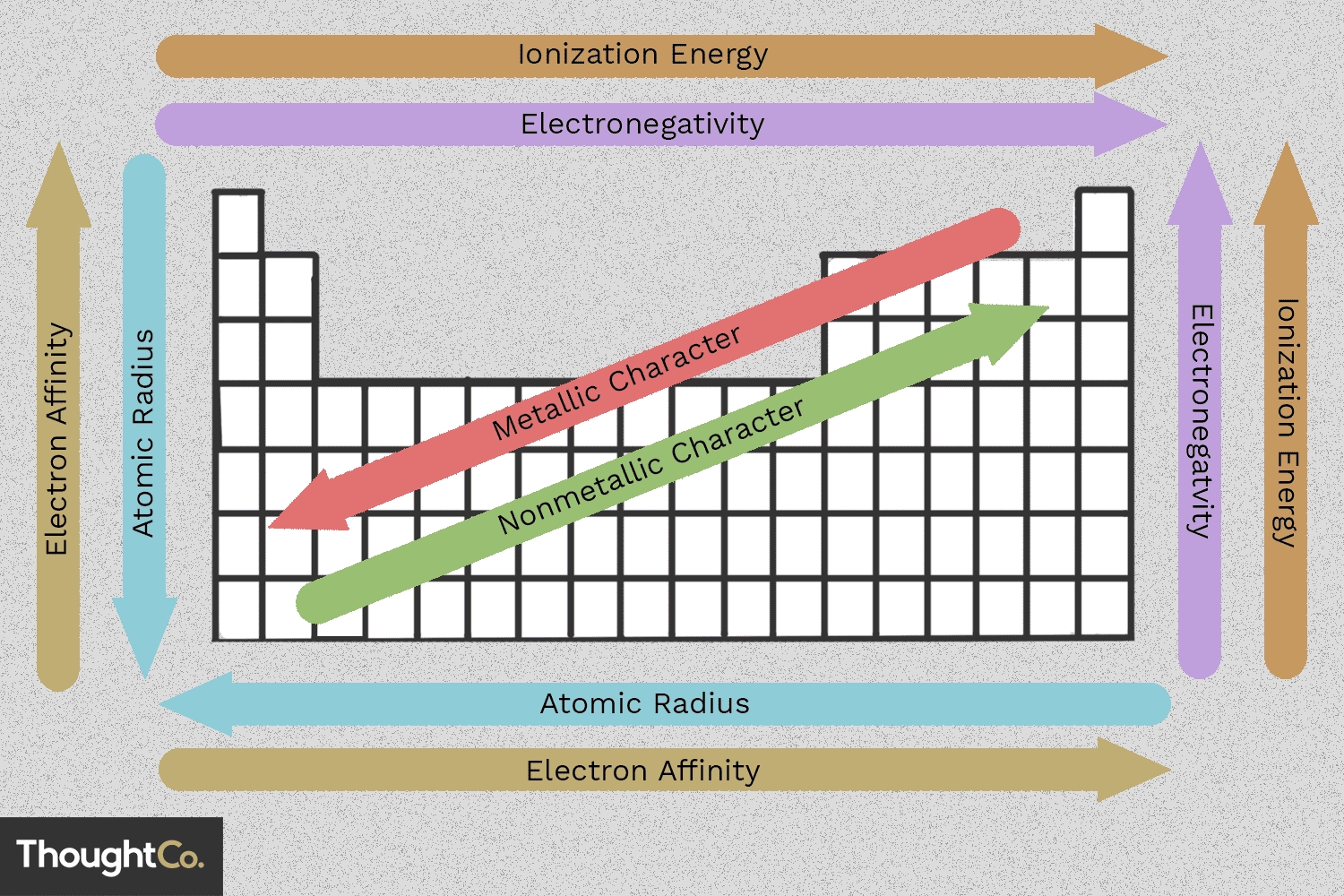
As we explore the periodic table, we discover that certain properties of elements tend to change in predictable ways as we move from one element to another. These changes are known as periodic trends. Understanding these trends helps us anticipate the behavior of elements and their compounds.
Atomic Radius
The atomic radius is the distance from the nucleus to the outermost electron in a neutral atom. As we move:
- Down a group: The atomic radius increases because each successive element has an additional energy level, resulting in a larger atomic size.
- Across a period: The atomic radius decreases because the effective nuclear charge increases, pulling the electrons closer to the nucleus.
| Period | Group 1 | Group 2 | Group 13 | Group 14 | Group 15 | Group 16 | Group 17 |
|---|---|---|---|---|---|---|---|
| 2 | 152 | 160 | 184 | 194 | 203 | 216 | 231 |
| 3 | 222 | 232 | 255 | 267 | 279 | 293 | 307 |
| 4 | 298 | 313 | 339 | 353 | 367 | 383 | 401 |
Note: The values in the table are in picometers (pm).
Ionic Radius
The ionic radius is the distance from the nucleus to the outermost electron in an ion. As we move:
- Down a group: The ionic radius increases because each successive element has an additional energy level, resulting in a larger ionic size.
- Across a period: The ionic radius decreases because the effective nuclear charge increases, pulling the electrons closer to the nucleus.
| Period | Group 1 | Group 2 | Group 13 | Group 14 | Group 15 | Group 16 | Group 17 |
|---|---|---|---|---|---|---|---|
| 2 | 95 | 110 | 125 | 140 | 155 | 170 | 185 |
| 3 | 135 | 150 | 165 | 180 | 195 | 210 | 225 |
| 4 | 180 | 195 | 210 | 225 | 240 | 255 | 270 |
Note: The values in the table are in picometers (pm).
Electronegativity
Electronegativity is a measure of an atom’s ability to attract electrons in a covalent bond. As we move:
- Down a group: Electronegativity decreases because the outermost electrons are farther away from the nucleus, resulting in a weaker attraction.
- Across a period: Electronegativity increases because the effective nuclear charge increases, resulting in a stronger attraction to electrons.
| Period | Group 1 | Group 2 | Group 13 | Group 14 | Group 15 | Group 16 | Group 17 |
|---|---|---|---|---|---|---|---|
| 2 | 0.93 | 1.20 | 1.47 | 1.74 | 2.01 | 2.28 | 2.55 |
| 3 | 0.86 | 1.10 | 1.35 | 1.60 | 1.85 | 2.10 | 2.35 |
| 4 | 0.79 | 1.00 | 1.23 | 1.46 | 1.69 | 1.92 | 2.15 |
Note: The values in the table are on the Pauling scale.
Ionization Energy
Ionization energy is the energy required to remove an electron from a neutral atom. As we move:
- Down a group: Ionization energy decreases because the outermost electrons are farther away from the nucleus, resulting in a lower energy requirement.
- Across a period: Ionization energy increases because the effective nuclear charge increases, resulting in a higher energy requirement.
| Period | Group 1 | Group 2 | Group 13 | Group 14 | Group 15 | Group 16 | Group 17 |
|---|---|---|---|---|---|---|---|
| 2 | 375 | 420 | 470 | 520 | 570 | 620 | 670 |
| 3 | 345 | 390 | 440 | 490 | 540 | 590 | 640 |
| 4 | 320 | 360 | 410 | 460 | 510 | 560 | 610 |
Note: The values in the table are in kilojoules per mole (kJ/mol).
By understanding these periodic trends, we can better comprehend the behavior of elements and their compounds. Remember to use these trends to make predictions and explain phenomena in chemistry.
To summarize:
- Atomic radius increases down a group and decreases across a period.
- Ionic radius increases down a group and decreases across a period.
- Electronegativity decreases down a group and increases across a period.
- Ionization energy decreases down a group and increases across a period.
What is the trend for atomic radius as we move down a group?
+
The atomic radius increases as we move down a group.
What is the trend for electronegativity as we move across a period?
+
Electronegativity increases as we move across a period.
What is the trend for ionization energy as we move down a group?
+
Ionic radius increases as we move down a group.