Master Periodic Trends in 5 Easy Steps
Understanding Periodic Trends: A Comprehensive Guide
The periodic table is a powerful tool for chemists and chemistry students, providing a wealth of information about the properties and behavior of elements. However, the sheer amount of data presented in the periodic table can be overwhelming, especially for those new to chemistry. Mastering periodic trends is essential to unlock the secrets of the periodic table and make predictions about the properties of elements. In this article, we will explore five easy steps to master periodic trends and take your chemistry skills to the next level.
Step 1: Understand the Structure of the Periodic Table
The periodic table is arranged in a logical and systematic way, with elements grouped into rows (periods) and columns (groups or families). The elements in each group exhibit similar chemical properties due to the same number of electrons in their outermost energy level. The periods are arranged in order of increasing atomic number, with elements in the same period exhibiting trends in their properties.
📝 Note: The periodic table is a dynamic tool, and its structure is constantly being refined and updated as new elements are discovered.
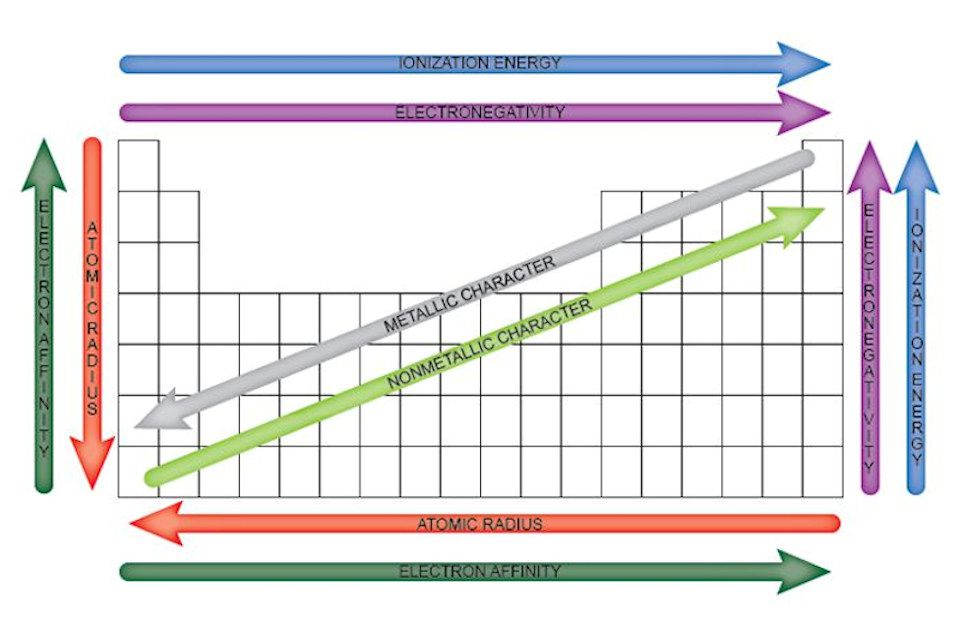
Step 2: Identify the Main Types of Periodic Trends
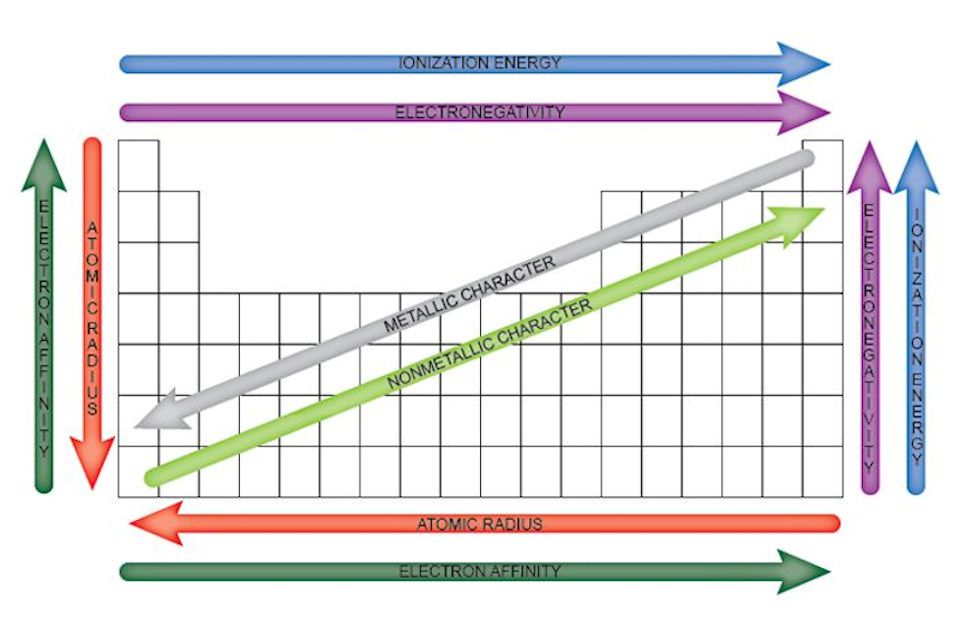
There are several types of periodic trends, including:
- Atomic radius: The distance from the nucleus to the outermost electron.
- Electronegativity: The ability of an atom to attract electrons in a covalent bond.
- Ionization energy: The energy required to remove an electron from an atom.
- Electron affinity: The energy released when an electron is added to an atom.
- Valence electrons: The number of electrons in an atom’s outermost energy level.
These trends are essential to understanding the properties and behavior of elements.
Step 3: Analyze Trends Across Periods and Groups
As you move across a period from left to right, the atomic radius decreases, and the electronegativity increases. This is because the number of protons in the nucleus increases, pulling the electrons closer to the nucleus. As you move down a group from top to bottom, the atomic radius increases, and the electronegativity decreases. This is because the number of energy levels increases, pushing the electrons further away from the nucleus.
📊 Note: Trends can be affected by other factors, such as the presence of transition metals or noble gases.
Step 4: Use Periodic Trends to Make Predictions
By understanding periodic trends, you can make predictions about the properties of elements. For example, if you know the atomic radius of an element, you can predict its electronegativity and reactivity. You can also use periodic trends to identify patterns and relationships between elements.
- Reactivity: Elements with high electronegativity tend to be more reactive.
- Ionization energy: Elements with low ionization energy tend to be more reactive.
- Electron affinity: Elements with high electron affinity tend to be more reactive.
Step 5: Practice and Apply Periodic Trends
The key to mastering periodic trends is practice and application. Try to:
- Identify trends: Look for patterns and relationships between elements.
- Make predictions: Use periodic trends to predict the properties of elements.
- Solve problems: Apply periodic trends to solve chemistry problems.
| Element | Atomic Radius (pm) | Electronegativity |
|---|---|---|
| Lithium (Li) | 152 | 0.98 |
| Beryllium (Be) | 112 | 1.57 |
| Boron (B) | 87 | 2.04 |
By following these five easy steps, you can master periodic trends and take your chemistry skills to the next level. Remember to practice and apply periodic trends to solve chemistry problems and make predictions about the properties of elements.
As you continue to explore the world of chemistry, you will find that mastering periodic trends is essential to unlocking the secrets of the periodic table. With practice and application, you can become proficient in using periodic trends to make predictions and solve chemistry problems.
What is the periodic table, and why is it important in chemistry?
+The periodic table is a tabular arrangement of elements, organized by their atomic number, electron configuration, and recurring chemical properties. It is essential in chemistry because it provides a systematic way to organize and predict the properties of elements.
What are periodic trends, and how do they relate to the periodic table?
+Periodic trends refer to the patterns and relationships between the properties of elements, as you move across periods and groups in the periodic table. These trends are essential to understanding the properties and behavior of elements.
How can I use periodic trends to make predictions about the properties of elements?
+By understanding periodic trends, you can make predictions about the properties of elements, such as their reactivity, electronegativity, and ionization energy. This can be done by analyzing the trends across periods and groups in the periodic table.