5 Easy Steps to Master Percent Yield Calculations
Understanding Percent Yield Calculations
Percent yield calculations are a fundamental concept in chemistry, and mastering them can help you tackle complex problems with ease. Whether you’re a student, researcher, or professional chemist, understanding percent yield is essential to measure the efficiency of chemical reactions. In this article, we’ll break down the concept of percent yield and provide a step-by-step guide to calculate it with ease.
What is Percent Yield?
Percent yield is a measure of the efficiency of a chemical reaction. It’s defined as the ratio of the actual yield of a product to the theoretical yield, expressed as a percentage. The theoretical yield is the maximum amount of product that can be obtained from a reaction, based on the stoichiometry of the reactants. The actual yield, on the other hand, is the amount of product actually obtained from the reaction.
Why is Percent Yield Important?
Percent yield is crucial in chemistry because it helps us evaluate the efficiency of a reaction. A high percent yield indicates that the reaction is efficient, while a low percent yield suggests that the reaction is not optimal. By calculating percent yield, chemists can identify areas for improvement, optimize reaction conditions, and scale up reactions for industrial applications.
5 Easy Steps to Master Percent Yield Calculations
Now that we’ve covered the basics, let’s dive into the step-by-step guide to calculating percent yield.
Step 1: Determine the Theoretical Yield
To calculate percent yield, you need to determine the theoretical yield of the reaction. This involves calculating the amount of product that can be obtained from the reaction, based on the stoichiometry of the reactants.
🔍 Note: Make sure you have a balanced equation for the reaction, as this will help you determine the stoichiometry of the reactants.
Step 2: Calculate the Actual Yield
Next, you need to calculate the actual yield of the reaction. This involves measuring the amount of product obtained from the reaction.
🔍 Note: Make sure you have accurate measurements of the product, as this will affect the accuracy of your percent yield calculation.
Step 3: Calculate the Percent Yield
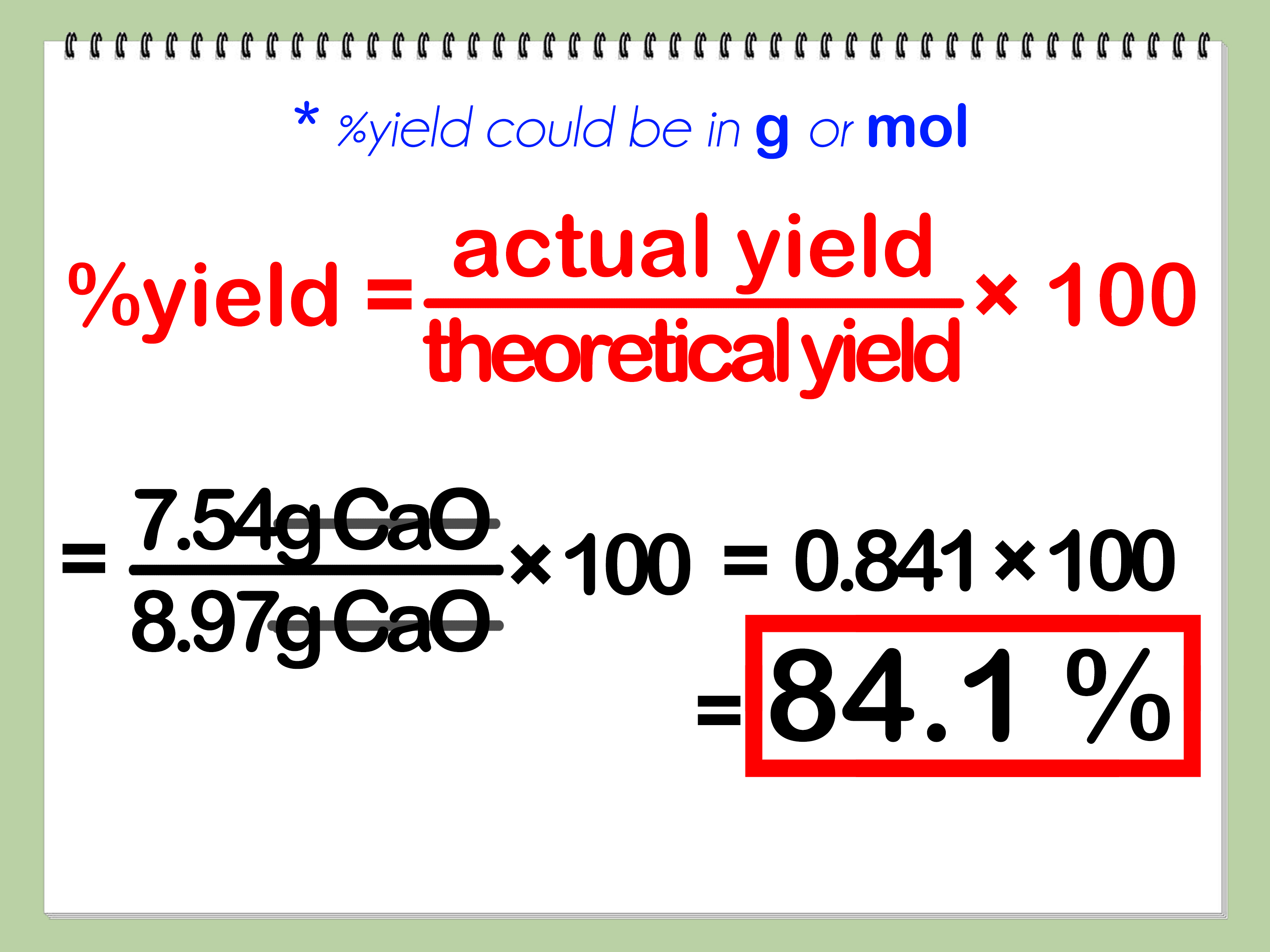
Now that you have the theoretical yield and actual yield, you can calculate the percent yield using the following formula:
Percent Yield = (Actual Yield / Theoretical Yield) x 100
Step 4: Evaluate the Percent Yield
Once you’ve calculated the percent yield, evaluate it to determine the efficiency of the reaction. A high percent yield indicates that the reaction is efficient, while a low percent yield suggests that the reaction is not optimal.
Step 5: Optimize the Reaction (Optional)
If the percent yield is low, you may need to optimize the reaction conditions to improve the efficiency of the reaction. This could involve adjusting the temperature, pressure, or concentration of reactants.
Example Calculation
Let’s say we’re conducting a reaction to produce a product, and we want to calculate the percent yield. We have the following data:
- Theoretical yield: 100 g
- Actual yield: 80 g
Using the formula, we can calculate the percent yield as follows:
Percent Yield = (80 g / 100 g) x 100 = 80%
This means that the reaction has an efficiency of 80%.
Conclusion
Mastering percent yield calculations is essential for chemists, researchers, and students. By following these 5 easy steps, you can calculate percent yield with ease and evaluate the efficiency of chemical reactions. Remember to always evaluate the percent yield and optimize the reaction conditions to improve the efficiency of the reaction.
What is the difference between theoretical yield and actual yield?
+Theoretical yield is the maximum amount of product that can be obtained from a reaction, based on the stoichiometry of the reactants. Actual yield, on the other hand, is the amount of product actually obtained from the reaction.
Why is percent yield important in chemistry?
+Percent yield is important in chemistry because it helps us evaluate the efficiency of a reaction. A high percent yield indicates that the reaction is efficient, while a low percent yield suggests that the reaction is not optimal.
How can I optimize the reaction conditions to improve the percent yield?
+To optimize the reaction conditions, you can try adjusting the temperature, pressure, or concentration of reactants. You can also try using different catalysts or solvents to improve the efficiency of the reaction.
Related Terms:
- Percent yield worksheet answers
- Worksheet percent yield
- Percent yield Activity
- Theoretical and percent yield Worksheet
- Percentage yield pdf