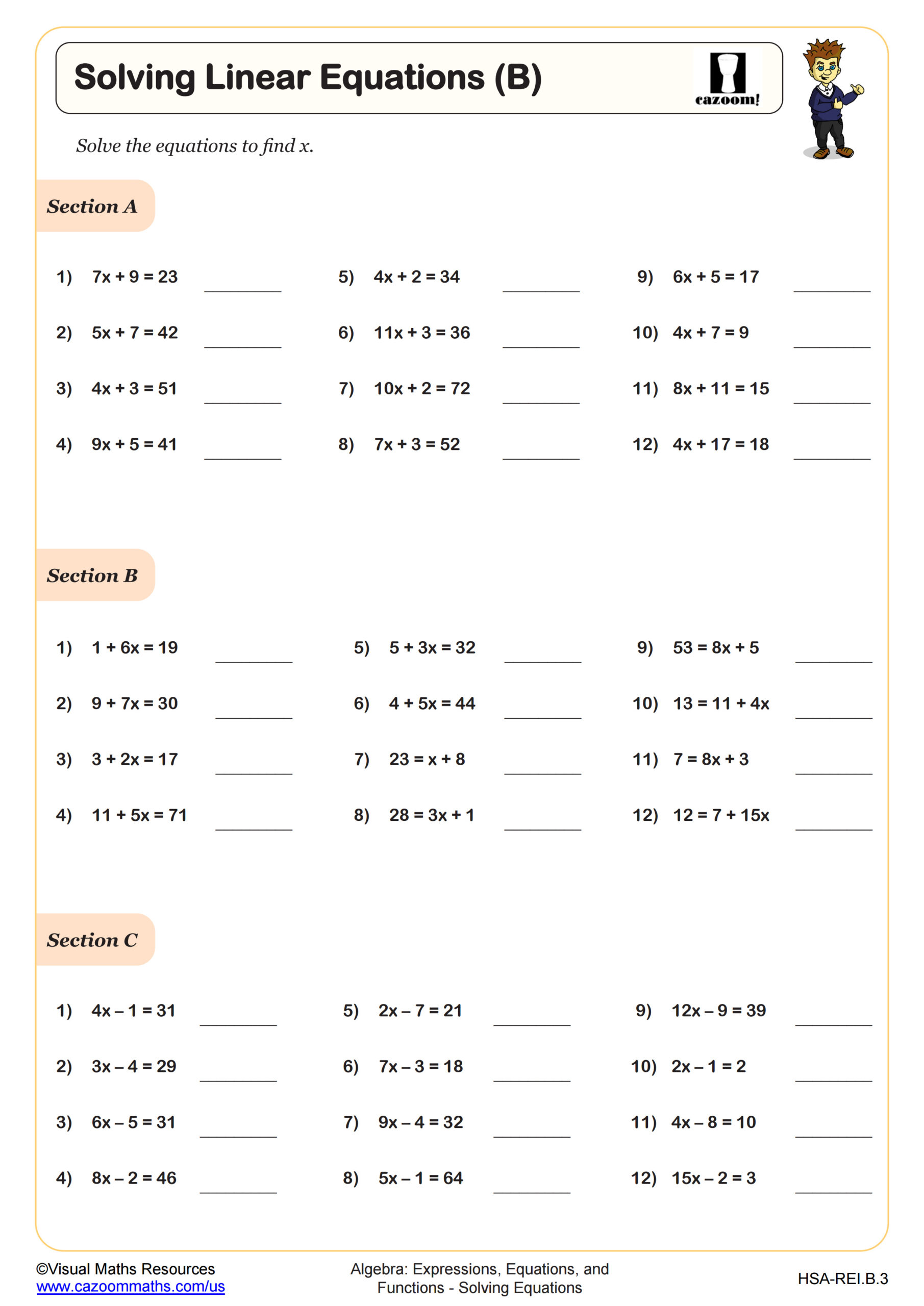
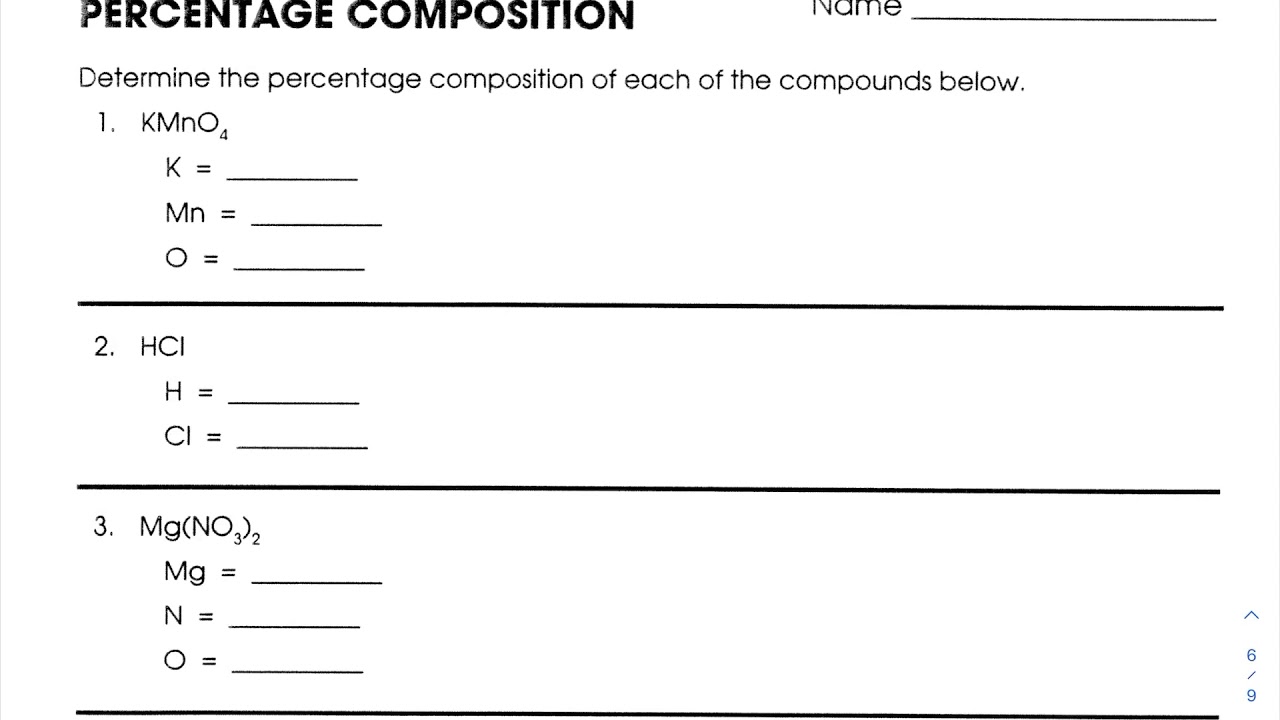
Percent Composition Worksheet Answer Key and Solutions

Understanding Percent Composition
Percent composition is a vital concept in chemistry that helps us understand the composition of a compound in terms of the percentage of each element present in it. It is defined as the mass percentage of each element in a compound and is calculated using the formula:
Percent Composition = (Mass of Element / Molar Mass of Compound) × 100
This concept is crucial in identifying the composition of unknown compounds and is used extensively in various fields such as chemistry, biochemistry, and materials science.
How to Calculate Percent Composition
Calculating percent composition involves a few simple steps:
- Determine the Molar Mass of the Compound: Calculate the molar mass of the compound by adding the atomic masses of all the atoms present in the compound.
- Calculate the Mass of Each Element: Calculate the mass of each element present in the compound by multiplying the atomic mass of the element by the number of atoms of that element present in the compound.
- Calculate the Percent Composition: Use the formula above to calculate the percent composition of each element.
Examples and Solutions
Let’s take a few examples to illustrate the calculation of percent composition:
Example 1: Calculate the Percent Composition of Water (H2O)
| Element | Atomic Mass | Number of Atoms | Mass of Element |
|---|---|---|---|
| H | 1.01 g/mol | 2 | 2.02 g/mol |
| O | 16.00 g/mol | 1 | 16.00 g/mol |
Molar Mass of Water = 2.02 + 16.00 = 18.02 g/mol
Percent Composition of H = (2.02 / 18.02) × 100 = 11.19% Percent Composition of O = (16.00 / 18.02) × 100 = 88.81%
Example 2: Calculate the Percent Composition of Carbon Dioxide (CO2)
| Element | Atomic Mass | Number of Atoms | Mass of Element |
|---|---|---|---|
| C | 12.01 g/mol | 1 | 12.01 g/mol |
| O | 16.00 g/mol | 2 | 32.00 g/mol |
Molar Mass of Carbon Dioxide = 12.01 + 32.00 = 44.01 g/mol
Percent Composition of C = (12.01 / 44.01) × 100 = 27.29% Percent Composition of O = (32.00 / 44.01) × 100 = 72.71%
Example 3: Calculate the Percent Composition of Glucose (C6H12O6)
| Element | Atomic Mass | Number of Atoms | Mass of Element |
|---|---|---|---|
| C | 12.01 g/mol | 6 | 72.06 g/mol |
| H | 1.01 g/mol | 12 | 12.12 g/mol |
| O | 16.00 g/mol | 6 | 96.00 g/mol |
Molar Mass of Glucose = 72.06 + 12.12 + 96.00 = 180.18 g/mol
Percent Composition of C = (72.06 / 180.18) × 100 = 40.00% Percent Composition of H = (12.12 / 180.18) × 100 = 6.72% Percent Composition of O = (96.00 / 180.18) × 100 = 53.28%
Important Notes
💡 Note: The atomic masses used in the calculations are approximate values and may vary slightly depending on the source.
📝 Note: The calculations are based on the assumption that the compounds are in their simplest form and do not contain any isotopes.
Conclusion
Calculating percent composition is a straightforward process that involves determining the molar mass of a compound and calculating the mass of each element present in the compound. By using the formula for percent composition, we can easily determine the percentage of each element in a compound. This concept is essential in understanding the composition of various substances and is widely used in various fields of science and engineering.
What is percent composition?
+Percent composition is the mass percentage of each element in a compound.
How is percent composition calculated?
+Percent composition is calculated using the formula: (Mass of Element / Molar Mass of Compound) × 100
What is the importance of percent composition?
+Percent composition is essential in understanding the composition of various substances and is widely used in various fields of science and engineering.