Percent Composition Practice Worksheet for Chemistry Success
Mastering Percent Composition: A Key to Chemistry Success
Percent composition is a fundamental concept in chemistry that represents the percentage of each element present in a compound. It is a crucial tool for chemists to determine the composition of unknown substances, identify the purity of materials, and calculate the quantities of reactants and products in chemical reactions. In this article, we will delve into the world of percent composition, exploring its significance, formulas, and applications, as well as providing practice worksheets to help you master this essential concept.
Understanding Percent Composition
Percent composition is defined as the percentage of each element present in a compound, expressed as a percentage of the total mass of the compound. It is calculated by dividing the mass of each element by the total mass of the compound and multiplying by 100.
📝 Note: Percent composition is also known as percentage composition or %CE.
Formulas for Calculating Percent Composition
There are two main formulas for calculating percent composition:
- Percent Composition Formula
%CE = (mass of element / molar mass of compound) × 100
Where: %CE = percent composition of the element mass of element = mass of the element in the compound molar mass of compound = total molar mass of the compound
- Empirical Formula Formula
%CE = (number of atoms of element × atomic mass of element) / (molar mass of compound)
Where: %CE = percent composition of the element number of atoms of element = number of atoms of the element in the compound atomic mass of element = atomic mass of the element molar mass of compound = total molar mass of the compound
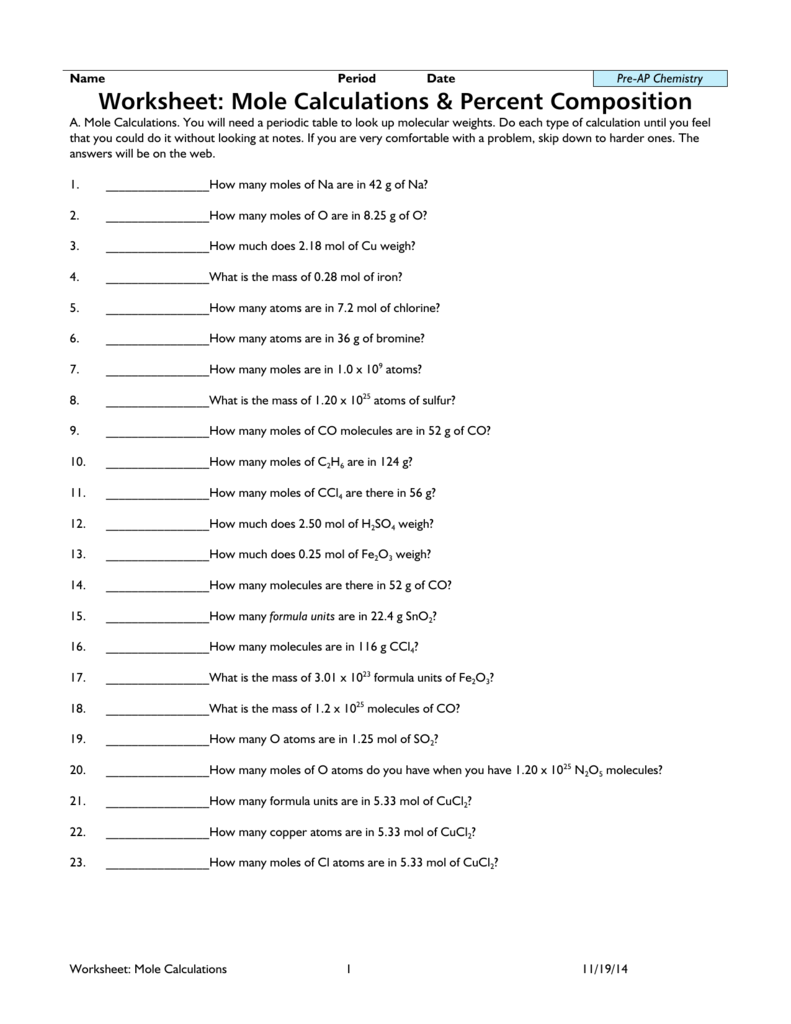
Practice Worksheet 1: Calculating Percent Composition
Calculate the percent composition of each element in the following compounds:

| Compound | Element | Mass of Element | Molar Mass of Compound |
|---|---|---|---|
| CO2 | Carbon | 12 g | 44 g/mol |
| CO2 | Oxygen | 32 g | 44 g/mol |
| H2O | Hydrogen | 2 g | 18 g/mol |
| H2O | Oxygen | 16 g | 18 g/mol |
| C6H12O6 | Carbon | 72 g | 180 g/mol |
| C6H12O6 | Hydrogen | 12 g | 180 g/mol |
| C6H12O6 | Oxygen | 96 g | 180 g/mol |
Practice Worksheet 2: Determining Empirical Formulas
Determine the empirical formula of each compound given the percent composition of each element:
| Compound | Element | %CE |
|---|---|---|
| A | Carbon | 40% |
| A | Hydrogen | 6.67% |
| A | Oxygen | 53.33% |
| B | Nitrogen | 30% |
| B | Oxygen | 70% |
| C | Calcium | 40% |
| C | Carbon | 12% |
| C | Oxygen | 48% |
Applications of Percent Composition
Percent composition has numerous applications in various fields, including:
- Chemical Analysis: Percent composition is used to determine the purity of materials, identify unknown substances, and analyze the composition of complex mixtures.
- Chemical Reactions: Percent composition is used to calculate the quantities of reactants and products in chemical reactions.
- Materials Science: Percent composition is used to determine the composition of materials, such as alloys and ceramics.
- Pharmaceuticals: Percent composition is used to determine the purity and composition of pharmaceuticals.
Conclusion
Mastering percent composition is essential for success in chemistry. By understanding the formulas, applications, and practice worksheets provided in this article, you will be well-equipped to tackle complex problems in chemistry. Remember to practice regularly and apply your knowledge to real-world scenarios to become proficient in percent composition.
What is percent composition?
+Percent composition is the percentage of each element present in a compound, expressed as a percentage of the total mass of the compound.
What are the two main formulas for calculating percent composition?
+The two main formulas are the percent composition formula and the empirical formula formula.
What is the significance of percent composition in chemistry?
+Percent composition is a fundamental concept in chemistry that has numerous applications in chemical analysis, chemical reactions, materials science, and pharmaceuticals.
Related Terms:
- Percent composition worksheet doc
- Percentage composition Worksheet answer KEY
- Percent composition word Problems Worksheet
- Empirical formula Worksheet