Empirical and Molecular Formulas Percent Composition Worksheet

Understanding Empirical and Molecular Formulas through Percent Composition
In chemistry, the composition of a molecule is crucial in understanding its properties and behavior. Two essential concepts in determining the composition of a molecule are empirical and molecular formulas. The empirical formula represents the simplest whole-number ratio of atoms of each element in a molecule, while the molecular formula shows the actual number of atoms of each element in a molecule. In this article, we will explore how to determine empirical and molecular formulas using percent composition, and provide a worksheet for practice.
What is Percent Composition?
Percent composition is the percentage of each element in a compound by mass. It is calculated by dividing the mass of each element in the compound by the total mass of the compound and multiplying by 100. The percent composition can be used to determine the empirical formula of a compound.
Determining Empirical Formulas from Percent Composition
To determine the empirical formula from percent composition, follow these steps:
- Assume a 100-gram sample of the compound.
- Convert the percent composition to grams.
- Divide each element’s mass by its atomic mass to get the number of moles.
- Divide each element’s number of moles by the smallest number of moles to get the simplest ratio.
- Write the empirical formula using the simplest ratio.
💡 Note: The empirical formula may not be the same as the molecular formula. The molecular formula can be determined using additional information, such as the molecular weight.
Determining Molecular Formulas from Empirical Formulas
To determine the molecular formula from the empirical formula, follow these steps:
- Calculate the molecular weight of the empirical formula.
- Divide the known molecular weight by the molecular weight of the empirical formula to get the multiplier.
- Multiply the subscripts in the empirical formula by the multiplier to get the molecular formula.
📝 Note: The molecular weight can be determined using various methods, such as mass spectrometry or gel permeation chromatography.
Worksheet: Empirical and Molecular Formulas from Percent Composition
Complete the following problems to practice determining empirical and molecular formulas from percent composition.
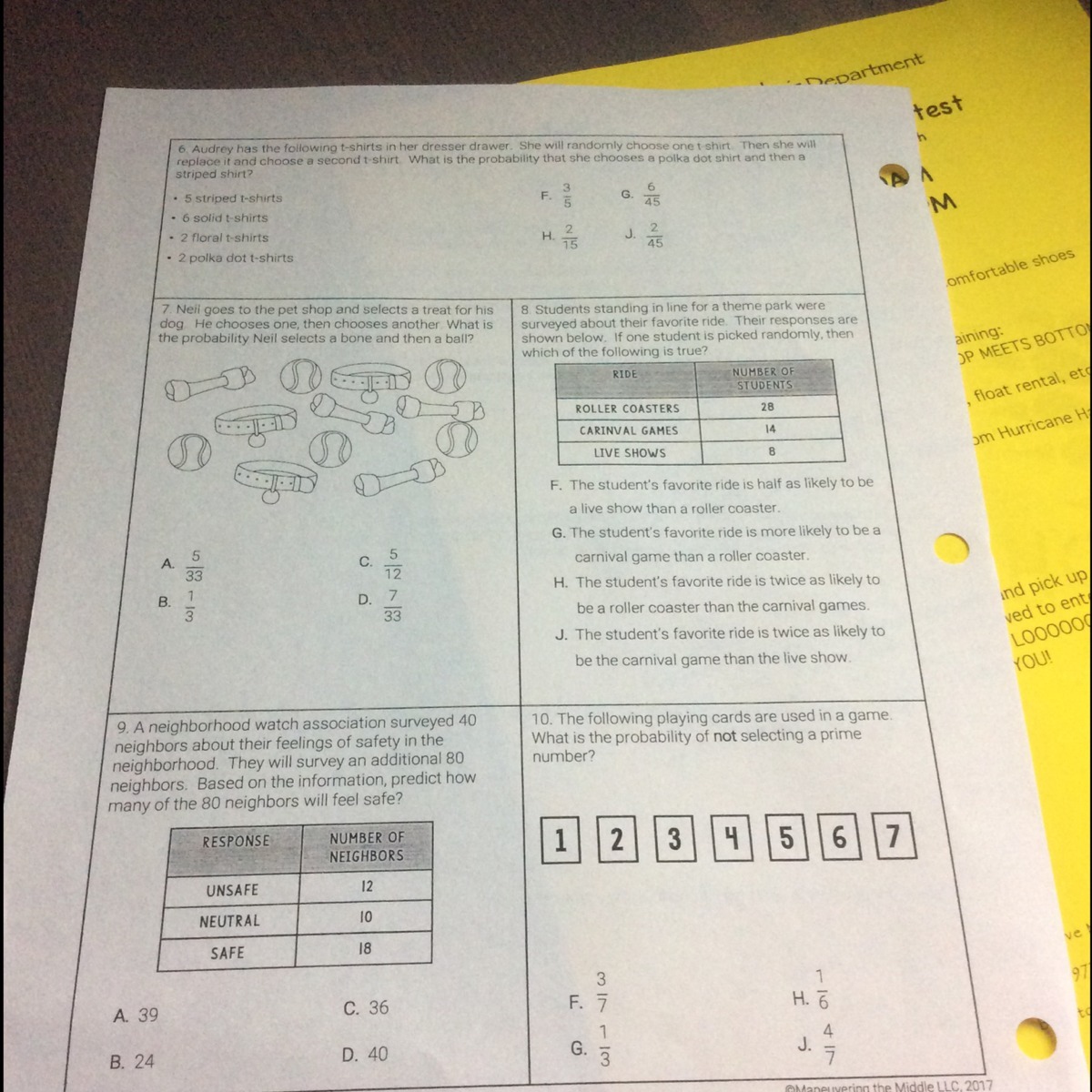
| Problem | Percent Composition | Empirical Formula | Molecular Formula |
|---|---|---|---|
| 1 | 40% C, 6.67% H, 53.33% O | ||
| 2 | 51.22% C, 13.11% H, 35.67% O | ||
| 3 | 29.81% N, 14.65% H, 55.54% O | ||
| 4 | 21.03% C, 4.57% H, 74.40% O | ||
| 5 | 65.45% C, 10.93% H, 23.62% O |
Answer Key
- Empirical Formula: CH₂O, Molecular Formula: C₂H₄O₂
- Empirical Formula: C₃H₄O, Molecular Formula: C₆H₈O₂
- Empirical Formula: N₂H₄O, Molecular Formula: N₂H₄O
- Empirical Formula: CH₂O, Molecular Formula: C₂H₄O₃
- Empirical Formula: C₄H₆O, Molecular Formula: C₈H₁₂O₂
In conclusion, determining empirical and molecular formulas from percent composition is an essential skill in chemistry. By following the steps outlined above and practicing with the worksheet, you can master this concept and apply it to various problems in chemistry.
What is the difference between empirical and molecular formulas?
+The empirical formula represents the simplest whole-number ratio of atoms of each element in a molecule, while the molecular formula shows the actual number of atoms of each element in a molecule.
How is percent composition used to determine empirical formulas?
+Percent composition is used to determine the empirical formula by converting the percent composition to grams, dividing each element’s mass by its atomic mass, and finding the simplest ratio of atoms.
What information is needed to determine molecular formulas from empirical formulas?
+The molecular weight of the compound is needed to determine the molecular formula from the empirical formula.