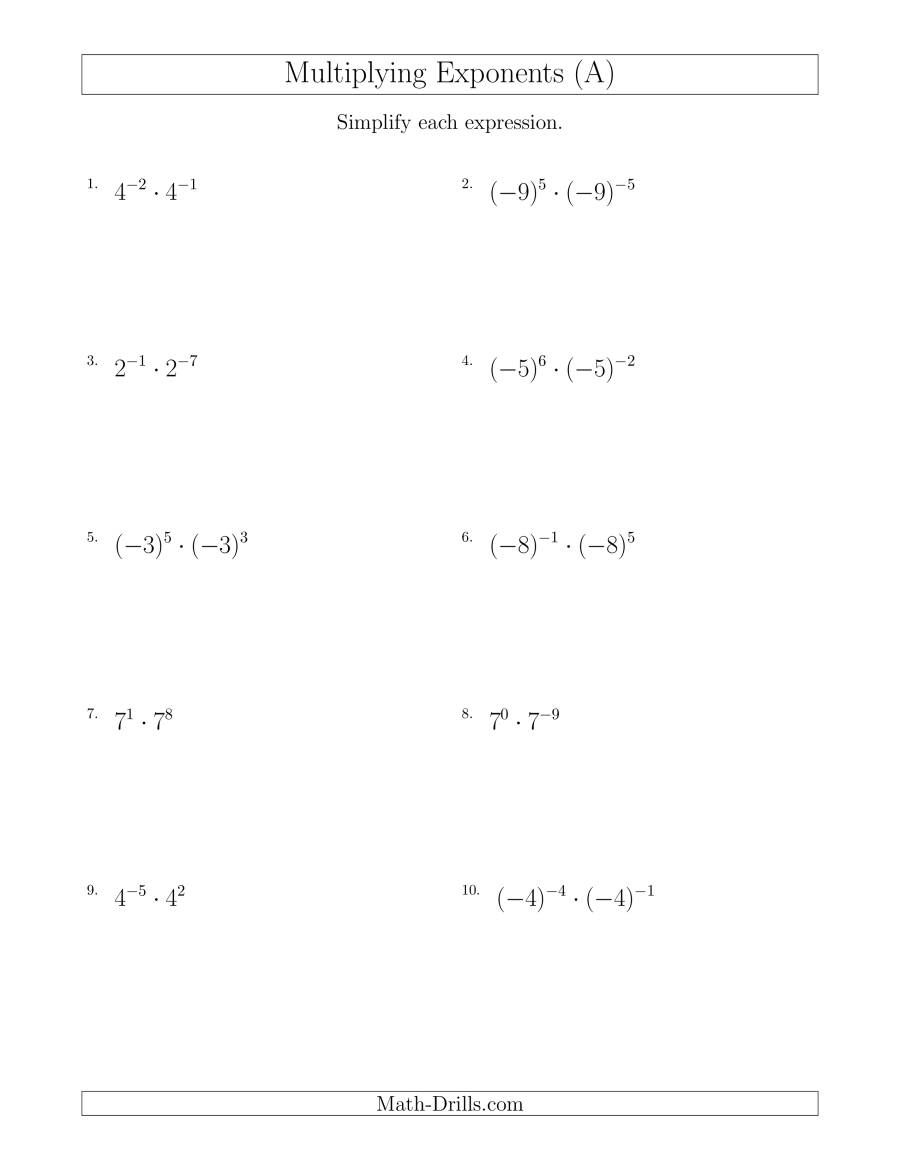Atom Basics: Understanding Parts of an Atom Worksheet
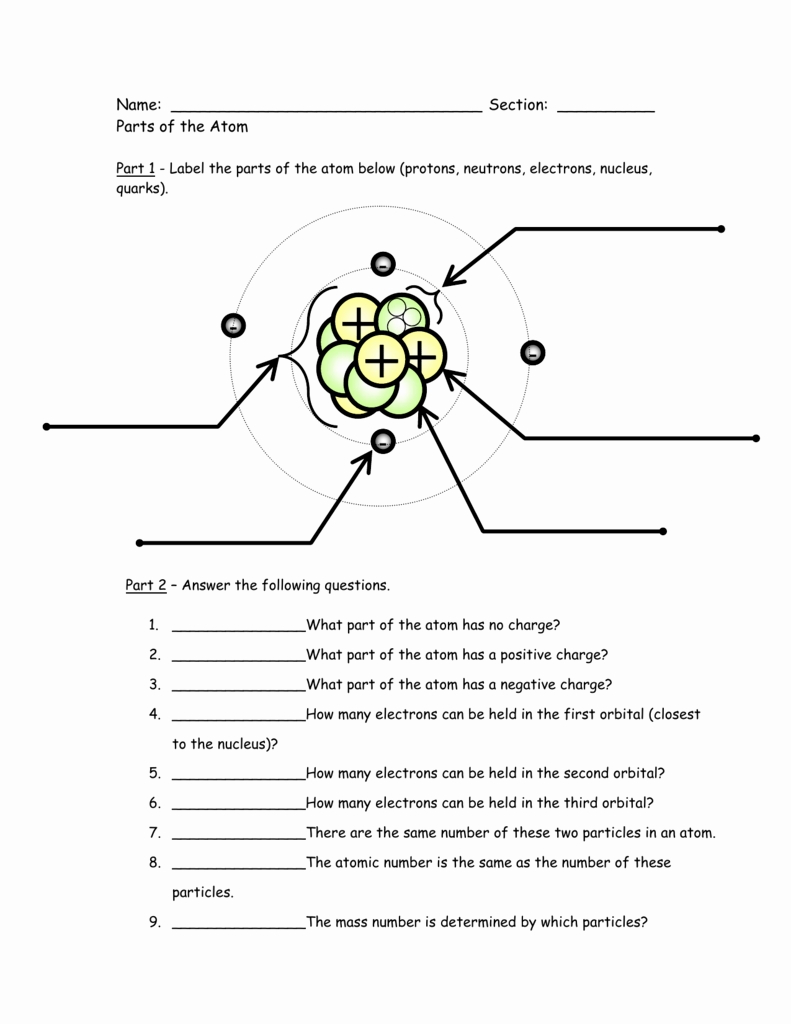
Introduction to Atom Basics
The study of atoms is a fundamental part of chemistry and physics. Atoms are the building blocks of matter, and understanding their structure and properties is essential for grasping many scientific concepts. In this article, we will explore the basics of atoms, including their parts and how they interact with each other.
What is an Atom?
An atom is the smallest unit of a chemical element that retains the properties of that element. Atoms are incredibly small, with diameters measured in picometers (trillionths of a meter). Despite their tiny size, atoms are the foundation of everything around us, from the air we breathe to the stars in the sky.
Parts of an Atom
An atom consists of three main parts: protons, neutrons, and electrons.
- Protons: Protons are positively charged particles that reside in the nucleus (center) of an atom. The number of protons in an atom determines the element of an atom, and each element has a unique number of protons in its atoms.
- Neutrons: Neutrons are particles that have no charge and are found in the nucleus along with protons. The number of neutrons in an atom can vary, and atoms of the same element can have different numbers of neutrons, leading to different isotopes.
- Electrons: Electrons are negatively charged particles that orbit the nucleus of an atom. The number of electrons in an atom is equal to the number of protons, and this number determines the chemical properties of an element.
Atomic Structure
The atomic structure refers to the arrangement of protons, neutrons, and electrons within an atom. The protons and neutrons are found in the nucleus, while the electrons occupy energy levels or electron shells around the nucleus. Each energy level can hold a specific number of electrons, and the arrangement of electrons in an atom determines its chemical properties.
Understanding Isotopes
Isotopes are atoms of the same element that have different numbers of neutrons. This variation in neutron number leads to differences in the physical properties of the isotopes, such as mass and radioactivity. Isotopes are commonly used in scientific research and have numerous applications in fields like medicine and industry.
Key Concepts and Terminology
- Atomic number: The number of protons in an atom, which determines the element.
- Mass number: The total number of protons and neutrons in an atom.
- Electron configuration: The arrangement of electrons in an atom.
- Isotope: Atoms of the same element with different numbers of neutrons.
🔬 Note: Understanding the basics of atoms is essential for grasping more advanced scientific concepts. Take your time to review and practice the concepts covered in this article.
Real-World Applications of Atomic Structure
The study of atomic structure has numerous real-world applications in fields like:
- Medicine: Radioisotopes are used in medical imaging and cancer treatment.
- Energy: Nuclear power plants generate electricity by harnessing the energy released from atomic reactions.
- Materials Science: Understanding atomic structure is crucial for the development of new materials with unique properties.
By grasping the basics of atoms and their structure, you can better appreciate the complexities of the scientific world and how they impact our daily lives.
Recap and Key Takeaways
To summarize, atoms are the building blocks of matter, consisting of protons, neutrons, and electrons. Understanding the parts of an atom and their arrangement is crucial for grasping scientific concepts and appreciating real-world applications.
Key takeaways:
- Atoms consist of protons, neutrons, and electrons.
- The number of protons determines the element, while the number of neutrons can vary.
- Electron configuration determines chemical properties.
- Isotopes have different numbers of neutrons, leading to variations in physical properties.
What is the smallest unit of a chemical element?
+The smallest unit of a chemical element is an atom.
What determines the chemical properties of an element?
+The arrangement of electrons in an atom determines its chemical properties.
What are isotopes?
+Isotopes are atoms of the same element with different numbers of neutrons.
The study of atoms and their structure is a fascinating field that underlies many scientific concepts. By understanding the basics of atoms, you can gain a deeper appreciation for the world around you and the incredible complexity of the scientific universe.
Related Terms:
- Parts of an atom pdf
- Parts of an atom Notes