Mastering Oxidation Numbers: A Step-by-Step Worksheet Guide
Understanding Oxidation Numbers: The Basics
Oxidation numbers, also known as oxidation states, are a fundamental concept in chemistry that helps us keep track of the transfer of electrons in chemical reactions. In this guide, we will walk you through the steps to master oxidation numbers, with a focus on how to assign, calculate, and use them to balance complex chemical equations.
What are Oxidation Numbers?
Oxidation numbers are a way to describe the number of electrons an atom has gained or lost in a chemical reaction. They are assigned to each atom in a molecule or ion, and can be either positive, negative, or zero. Oxidation numbers help us to:
- Identify the oxidation state of an atom
- Determine the number of electrons transferred in a reaction
- Balance complex chemical equations
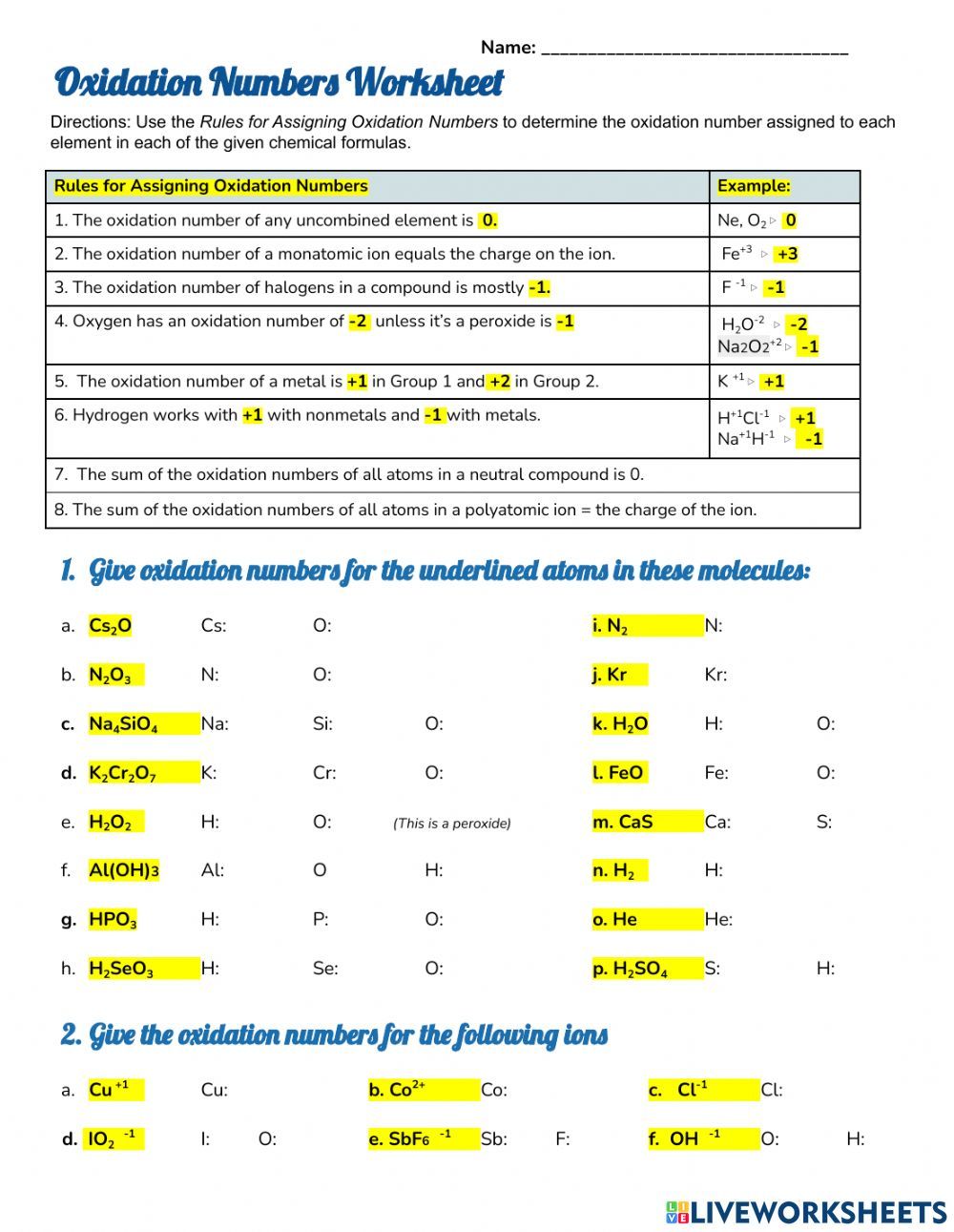
Rules for Assigning Oxidation Numbers
To assign oxidation numbers, follow these simple rules:
- Free elements: The oxidation number of a free element is always 0.
- Monatomic ions: The oxidation number of a monatomic ion is equal to its charge.
- Oxygen: The oxidation number of oxygen is usually -2, except in peroxides where it is -1.
- Hydrogen: The oxidation number of hydrogen is usually +1, except in hydrides where it is -1.
- Halides: The oxidation number of halides (F, Cl, Br, I) is usually -1, except when combined with oxygen or fluorine.
- Transition metals: The oxidation number of transition metals can vary, but is often +2 or +3.
How to Calculate Oxidation Numbers
To calculate the oxidation number of an atom in a molecule or ion, follow these steps:
- Identify the atoms in the molecule or ion.
- Assign the oxidation number to each atom using the rules above.
- Calculate the total oxidation number of the molecule or ion by summing the oxidation numbers of each atom.
Example: Calculate the oxidation number of the sulfur atom in the sulfate ion (SO42-).
- Identify the atoms: S, O, O, O, O.
- Assign the oxidation numbers: S =?, O = -2, O = -2, O = -2, O = -2.
- Calculate the total oxidation number: S + (-2 x 4) = -2 (since the sulfate ion has a charge of -2).
Solving for S, we get: S = +6.
Therefore, the oxidation number of the sulfur atom in the sulfate ion is +6.
Using Oxidation Numbers to Balance Chemical Equations
Oxidation numbers can be used to balance complex chemical equations by identifying the number of electrons transferred in a reaction.
Example: Balance the equation for the combustion of methane:
CH4 + O2 → CO2 + H2O
- Identify the atoms: C, H, H, H, H, O, O, C, O, O, H, H.
- Assign the oxidation numbers: C = -4, H = +1, O = -2, C = +4, O = -2, H = +1.
- Calculate the total oxidation number: -4 + (4 x +1) + (-2 x 2) = 0 (since the reactants and products have the same number of electrons).
To balance the equation, we need to add electrons to the reactants or products to equalize the oxidation numbers. In this case, we add 4 electrons to the oxygen atoms on the reactant side:
CH4 + 2O2 → CO2 + 2H2O
Table: Common Oxidation Numbers of Elements
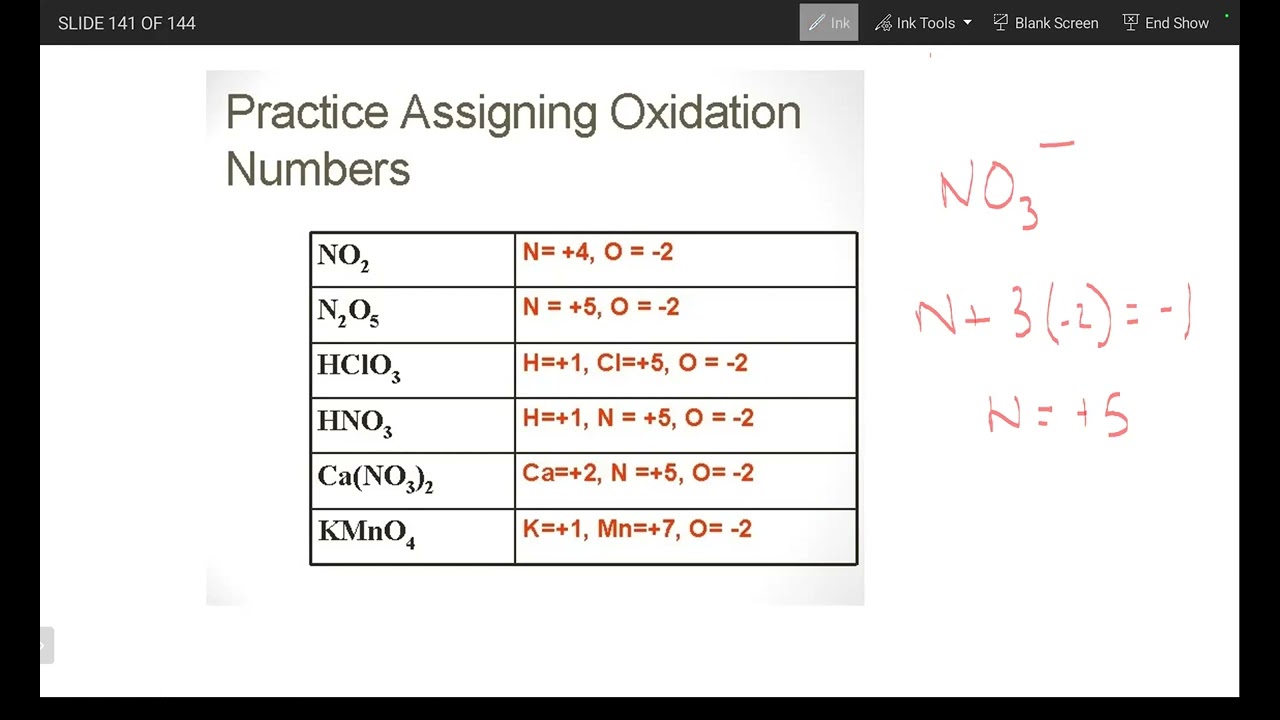
| Element | Oxidation Number |
|---|---|
| Hydrogen | +1, -1 |
| Oxygen | -2, -1 |
| Nitrogen | -3, +3, +5 |
| Sulfur | -2, +4, +6 |
| Carbon | -4, +2, +4 |
| Halides (F, Cl, Br, I) | -1 |
💡 Note: This table is not exhaustive, but it covers the most common elements and their oxidation numbers.
Common Mistakes to Avoid
When working with oxidation numbers, be careful to avoid these common mistakes:
- Misassigning oxidation numbers: Make sure to follow the rules for assigning oxidation numbers, and double-check your work.
- Forgetting to calculate the total oxidation number: Always calculate the total oxidation number of the molecule or ion to ensure accuracy.
- Not using oxidation numbers to balance chemical equations: Oxidation numbers can be a powerful tool for balancing complex chemical equations. Don’t neglect to use them!
What is the oxidation number of oxygen in a peroxide?
+The oxidation number of oxygen in a peroxide is -1.
How do I calculate the oxidation number of an atom in a molecule or ion?
+To calculate the oxidation number of an atom, identify the atoms in the molecule or ion, assign the oxidation number to each atom using the rules above, and calculate the total oxidation number of the molecule or ion by summing the oxidation numbers of each atom.
Why are oxidation numbers important in chemistry?
+Oxidation numbers help us to identify the oxidation state of an atom, determine the number of electrons transferred in a reaction, and balance complex chemical equations.
In conclusion, mastering oxidation numbers is a crucial skill for any chemistry student or professional. By following the rules for assigning oxidation numbers, calculating the total oxidation number of a molecule or ion, and using oxidation numbers to balance chemical equations, you can gain a deeper understanding of the chemistry underlying complex reactions. Remember to avoid common mistakes, such as misassigning oxidation numbers or forgetting to calculate the total oxidation number, and use the table of common oxidation numbers as a reference guide.