Osmosis and Tonicity Worksheet Answers and Study Guide
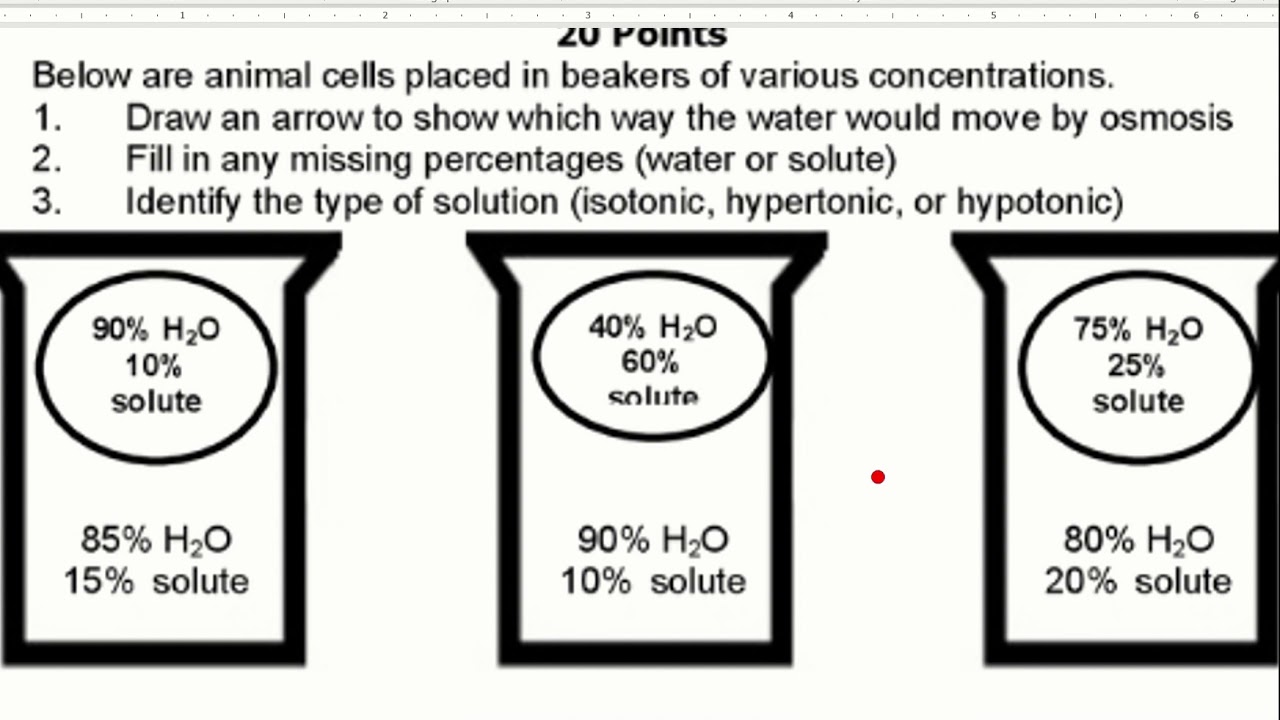
Osmosis and Tonicity: A Comprehensive Study Guide
Osmosis and tonicity are two fundamental concepts in biology that help us understand how cells interact with their environment. In this study guide, we will delve into the world of osmosis and tonicity, exploring their definitions, types, and significance in various biological processes.
What is Osmosis?
Osmosis is the movement of water molecules from an area of high concentration to an area of low concentration through a selectively permeable membrane. This process helps to equalize the concentration of solutes on both sides of the membrane. Osmosis is essential for maintaining cellular homeostasis, as it allows cells to regulate their internal environment and maintain proper ion balance.
Types of Osmosis
There are three types of osmosis:
- Endosmosis: The movement of water into a cell, resulting in an increase in cell size.
- Exosmosis: The movement of water out of a cell, resulting in a decrease in cell size.
- Isotonic: The movement of water into and out of a cell, resulting in no net change in cell size.
What is Tonicity?
Tonicity refers to the relative concentration of solutes in a solution compared to the concentration of solutes in a cell. A solution can be hypotonic (having a lower concentration of solutes than the cell), isotonic (having the same concentration of solutes as the cell), or hypertonic (having a higher concentration of solutes than the cell).
Types of Tonicity
There are three types of tonicity:
- Hypotonic: A solution with a lower concentration of solutes than the cell, resulting in an influx of water into the cell.
- Isotonic: A solution with the same concentration of solutes as the cell, resulting in no net movement of water.
- Hypertonic: A solution with a higher concentration of solutes than the cell, resulting in an efflux of water out of the cell.
Osmosis and Tonicity Worksheet Answers
Here are the answers to some common osmosis and tonicity worksheet questions:

| Question | Answer |
|---|---|
| What is the primary function of osmosis in cells? | Osmosis helps to regulate the concentration of solutes and maintain cellular homeostasis. |
| What type of osmosis occurs when a cell is placed in a hypotonic solution? | Endosmosis |
| What type of tonicity occurs when a solution has the same concentration of solutes as the cell? | Isotonic |
| What happens to a cell when it is placed in a hypertonic solution? | The cell shrinks due to the loss of water. |
| What is the term for a solution that has a lower concentration of solutes than the cell? | Hypotonic |
📝 Note: Osmosis and tonicity are critical concepts in biology, and understanding their mechanisms and types is essential for grasping various biological processes.
Importance of Osmosis and Tonicity
Osmosis and tonicity play crucial roles in various biological processes, including:
- Cellular homeostasis: Osmosis helps to regulate the concentration of solutes and maintain cellular homeostasis.
- Transport of nutrients and waste: Osmosis facilitates the movement of nutrients and waste products across cell membranes.
- Maintenance of proper ion balance: Tonicity helps to regulate the concentration of ions in the body, which is essential for proper cellular function.
Conclusion
In conclusion, osmosis and tonicity are essential concepts in biology that help us understand how cells interact with their environment. By understanding the mechanisms and types of osmosis and tonicity, we can appreciate the importance of these processes in maintaining cellular homeostasis and regulating various biological processes.
What is the difference between osmosis and tonicity?
+Osmosis refers to the movement of water molecules from an area of high concentration to an area of low concentration through a selectively permeable membrane. Tonicity, on the other hand, refers to the relative concentration of solutes in a solution compared to the concentration of solutes in a cell.
What type of tonicity occurs when a solution has the same concentration of solutes as the cell?
+Isotonic
What happens to a cell when it is placed in a hypotonic solution?
+The cell swells due to the influx of water.