Nuclear Chemistry Worksheet: Exploring Radioactive Reactions
Introduction to Nuclear Chemistry
Nuclear chemistry is a branch of chemistry that deals with the study of changes that occur within the nucleus of an atom. This field of study involves the analysis of radioactive reactions, nuclear stability, and the properties of radioactive isotopes. In this worksheet, we will explore the basics of nuclear chemistry and delve into the world of radioactive reactions.
What is Radioactivity?
Radioactivity is the process by which an unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves. This phenomenon was first discovered by Henri Becquerel in 1896, and since then, it has been extensively studied in various fields of science.
⚠️ Note: Radioactivity is a natural process that occurs in the environment, and it is also used in various applications, including medicine, industry, and scientific research.
Types of Radioactive Reactions
There are several types of radioactive reactions, including:
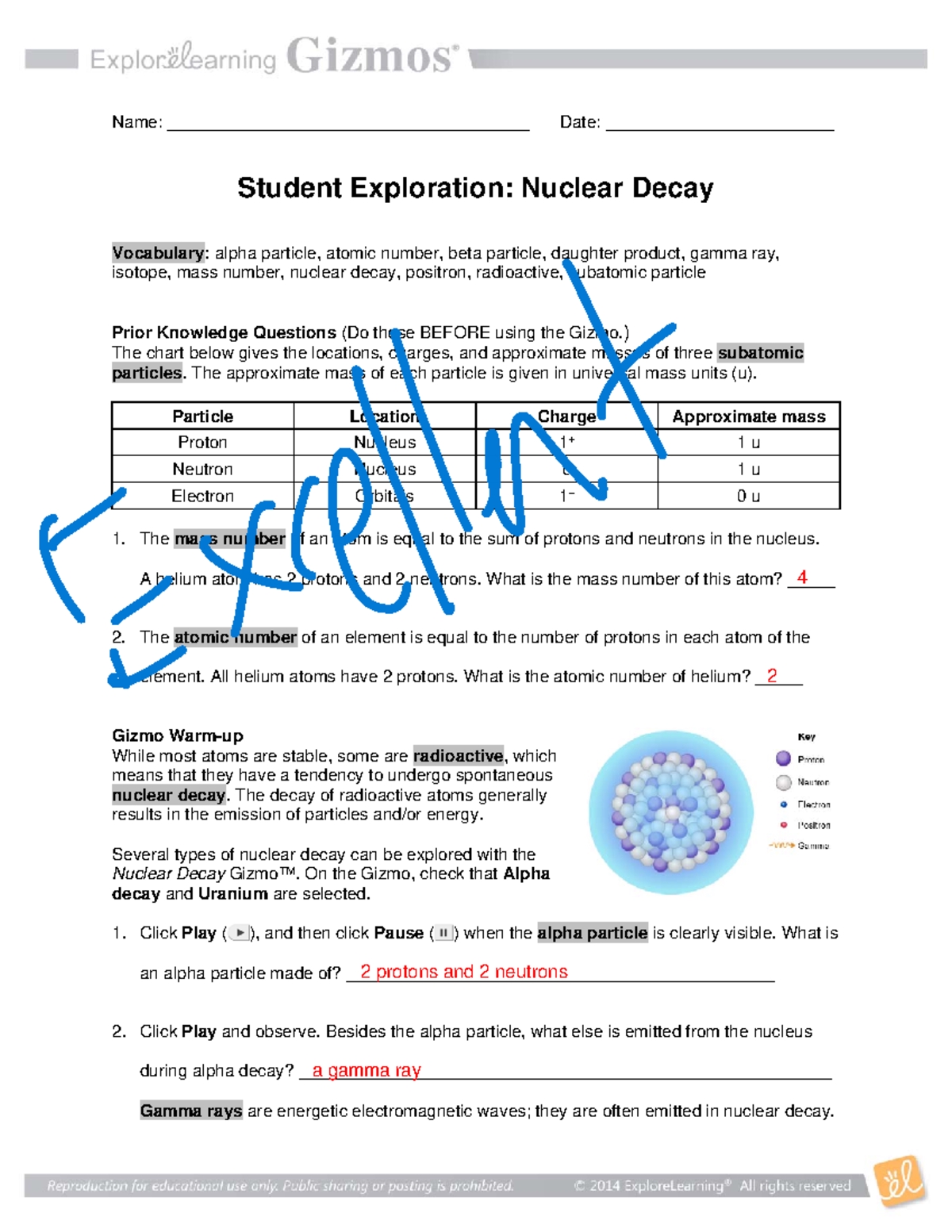
- Alpha Decay: The emission of an alpha particle (2 protons and 2 neutrons) from the nucleus of an atom.
- Beta Decay: The emission of a beta particle (an electron or a positron) from the nucleus of an atom.
- Gamma Decay: The emission of gamma radiation (high-energy electromagnetic waves) from the nucleus of an atom.
- Positron Emission: The emission of a positron (the antiparticle of an electron) from the nucleus of an atom.
Radioactive Decay Series
A radioactive decay series is a sequence of radioactive reactions that occur in a series of steps. Each step involves the emission of radiation, resulting in the transformation of one radioactive isotope into another.

| Isotope | Half-Life | Decay Mode | Daughter Isotope |
|---|---|---|---|
| Uranium-238 | 4.5 billion years | Alpha Decay | Thorium-234 |
| Thorium-234 | 24.1 days | Beta Decay | Protactinium-234 |
| Protactinium-234 | 1.17 minutes | Beta Decay | Uranium-234 |
Nuclear Stability
Nuclear stability refers to the stability of an atomic nucleus against radioactive decay. A nucleus is considered stable if it does not undergo radioactive decay.
🔑 Note: The stability of a nucleus depends on the number of protons and neutrons it contains. A nucleus with too many or too few neutrons is unstable and will undergo radioactive decay.
Applications of Radioactive Reactions
Radioactive reactions have several applications in various fields, including:
- Medicine: Radioisotopes are used in medical imaging and cancer treatment.
- Industry: Radioisotopes are used in food irradiation, sterilization of medical instruments, and in the production of plastics and semiconductors.
- Scientific Research: Radioisotopes are used in various scientific research applications, including geology, biology, and physics.
What is radioactivity?
+Radioactivity is the process by which an unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves.
What are the types of radioactive reactions?
+There are several types of radioactive reactions, including alpha decay, beta decay, gamma decay, and positron emission.
What is nuclear stability?
+Nuclear stability refers to the stability of an atomic nucleus against radioactive decay. A nucleus is considered stable if it does not undergo radioactive decay.
In conclusion, nuclear chemistry is a fascinating field that deals with the study of changes that occur within the nucleus of an atom. Radioactive reactions are an essential part of nuclear chemistry, and understanding these reactions is crucial for various applications in medicine, industry, and scientific research.
Related Terms:
- Kimia
- Kimia organik
- Kimia fisik
- Biokimia
- Kimia nuklir
- Nuclear Chemistry Worksheet answers pdf