5 Easy Ways to Master Naming Molecular Compounds
Mastering the art of naming molecular compounds is a crucial skill for any chemistry student. It can seem daunting at first, but with practice and the right strategies, you can become a pro in no time. In this article, we will explore five easy ways to master naming molecular compounds.
Understanding the Basics
Before we dive into the five easy ways, it’s essential to understand the basics of naming molecular compounds. Molecular compounds are formed when two or more nonmetal atoms share electrons to form a covalent bond. The naming convention for molecular compounds is based on the number of atoms of each element present in the compound.
📝 Note: The International Union of Pure and Applied Chemistry (IUPAC) is responsible for developing the rules for naming chemical compounds.
1. Learn the Prefixes
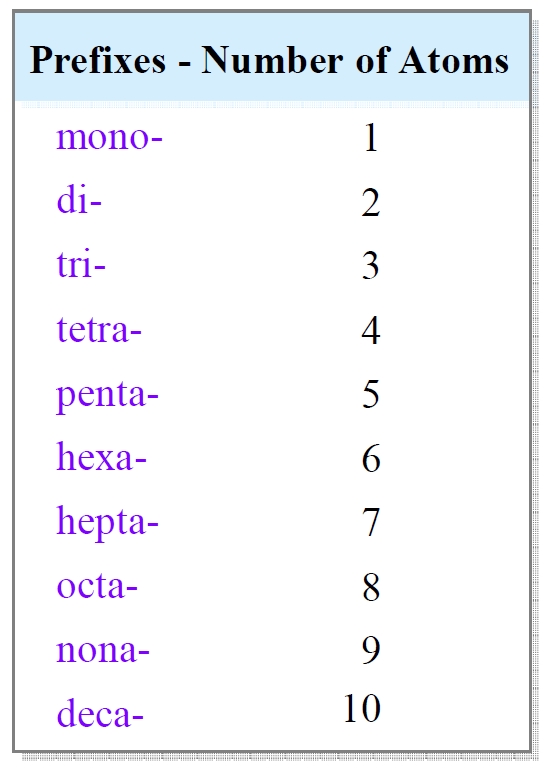
One of the easiest ways to master naming molecular compounds is to learn the prefixes. Prefixes are used to indicate the number of atoms of each element present in the compound. Here are the most common prefixes:
- Mono- (one atom)
- Di- (two atoms)
- Tri- (three atoms)
- Tetra- (four atoms)
- Penta- (five atoms)
- Hexa- (six atoms)
- Hepta- (seven atoms)
- Octa- (eight atoms)
- Nona- (nine atoms)
- Deca- (ten atoms)
For example, if you have a compound with two oxygen atoms, you would use the prefix di- to indicate the number of oxygen atoms.
2. Understand the Suffixes
Suffixes are used to indicate the type of compound. Here are the most common suffixes:
- -ide (indicates a binary compound)
- -ite (indicates a compound with a lower oxidation state)
- -ate (indicates a compound with a higher oxidation state)
- -ine (indicates a compound with a nitrogen atom)
For example, if you have a compound with a nitrogen atom, you would use the suffix -ine to indicate the presence of nitrogen.
3. Practice, Practice, Practice
Practice is key to mastering the art of naming molecular compounds. Try naming different compounds using the prefixes and suffixes you have learned. You can use online resources or textbooks to practice naming compounds.
Here are a few examples to get you started:
- CO (carbon monoxide)
- CO2 (carbon dioxide)
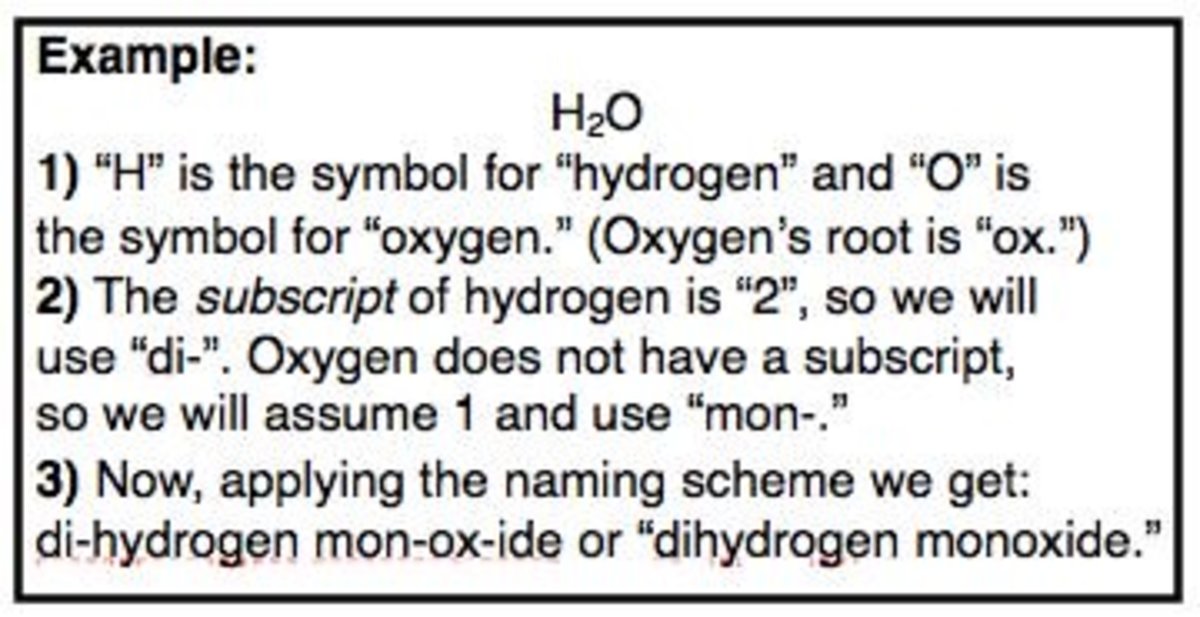
- H2O (water)
- NH3 (ammonia)
- CH4 (methane)
4. Use a Naming Flowchart
A naming flowchart can be a helpful tool to master naming molecular compounds. A flowchart can help you to visualize the naming process and ensure that you don’t miss any steps.
Here is a simple flowchart you can use:
| Step 1 | Step 2 | Step 3 |
|---|---|---|
| Identify the elements present in the compound | Determine the number of atoms of each element | Use prefixes and suffixes to name the compound |
5. Learn the Common Names
Finally, it’s essential to learn the common names of molecular compounds. Many compounds have common names that are widely used in industry and everyday life.
For example, water (H2O) is a common name for a compound that is widely used in everyday life. Methane (CH4) is another example of a compound with a common name.
By learning the common names of molecular compounds, you can master naming compounds and improve your knowledge of chemistry.
In summary, mastering the art of naming molecular compounds requires practice, patience, and dedication. By learning the prefixes, suffixes, and common names, you can become a pro at naming compounds in no time.
Mastering the art of naming molecular compounds is a crucial skill for any chemistry student. By following these five easy ways, you can improve your knowledge of chemistry and become a pro at naming compounds.
What is the difference between a molecular compound and an ionic compound?
+A molecular compound is a compound that is formed when two or more nonmetal atoms share electrons to form a covalent bond. An ionic compound is a compound that is formed when a metal atom loses electrons to form a positive ion, and a nonmetal atom gains electrons to form a negative ion.
What is the IUPAC rule for naming molecular compounds?
+The IUPAC rule for naming molecular compounds is to use prefixes and suffixes to indicate the number of atoms of each element present in the compound.
What is the prefix for one atom of an element?
+The prefix for one atom of an element is mono-.