5 Steps to Master Naming Ionic Compounds
Understanding the Basics of Ionic Compounds
Ionic compounds are formed when one or more electrons are transferred between atoms, resulting in the formation of ions with opposite charges. The electrostatic attraction between these oppositely charged ions holds them together, creating a strong chemical bond. Mastering the naming of ionic compounds is essential for chemistry students, as it helps to identify and communicate the composition of these compounds effectively. In this article, we will outline the 5 steps to master naming ionic compounds.
Step 1: Identify the Cations and Anions
The first step in naming an ionic compound is to identify the cations and anions present in the compound. Cations are positively charged ions, typically formed by metals, while anions are negatively charged ions, often formed by nonmetals. To identify the cations and anions, you need to be familiar with the periodic table and the common ions formed by different elements.
Common Cations:
- Alkali metals (Group 1): Li+, Na+, K+, Rb+, Cs+
- Alkaline earth metals (Group 2): Be2+, Mg2+, Ca2+, Sr2+, Ba2+
- Transition metals (Group 3-12): Fe2+, Fe3+, Cu2+, Zn2+, etc.
Common Anions:
- Halides (Group 17): F-, Cl-, Br-, I-
- Oxides (Group 16): O2-, S2-, Se2-, Te2-
- Nitrides (Group 15): N3-, P3-, As3-, Sb3-
Step 2: Determine the Charges of the Ions
Once you have identified the cations and anions, you need to determine their charges. The charge of an ion is determined by the number of electrons gained or lost during the formation of the ion. The charges of the ions must be balanced to form a neutral compound.
Tips for Determining Charges:
- Monatomic ions (single-atom ions) typically have a charge of 1- or 2- for anions and 1+ or 2+ for cations.
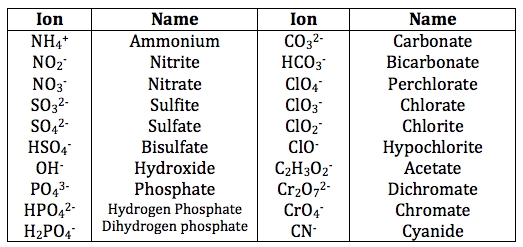
- Polyatomic ions (multi-atom ions) have a fixed charge, such as NO3- (nitrate), SO42- (sulfate), and PO43- (phosphate).
Step 3: Combine the Cation and Anion Names
To name an ionic compound, you combine the name of the cation and anion. The cation name is always written first, followed by the anion name.
Examples:
- Na+ (sodium) and Cl- (chloride) form NaCl (sodium chloride)
- Ca2+ (calcium) and O2- (oxide) form CaO (calcium oxide)
- Fe3+ (iron) and NO3- (nitrate) form Fe(NO3)3 (iron(III) nitrate)
Step 4: Use Roman Numerals for Transition Metals
Transition metals can form ions with different charges, so it’s essential to indicate the charge using Roman numerals.
Examples:
- Fe2+ (iron(II)) and Fe3+ (iron(III))
- Cu+ (copper(I)) and Cu2+ (copper(II))
Step 5: Check for Prefixes and Suffixes
Some ions have prefixes or suffixes that indicate their charge or composition. For example, the prefix “hypo-” indicates a lower charge, while the suffix “-ite” indicates a lower oxidation state.
Examples:
- Hypochlorite (ClO-) has a lower charge than chloride (Cl-)
- Sulfite (SO32-) has a lower oxidation state than sulfate (SO42-)
[📝] Note: When naming ionic compounds, it's essential to be consistent in your notation and to check for any prefixes or suffixes that may affect the name.
Conclusion
Mastering the naming of ionic compounds requires practice and attention to detail. By following these 5 steps, you can confidently name ionic compounds and improve your understanding of chemistry. Remember to identify the cations and anions, determine their charges, combine the cation and anion names, use Roman numerals for transition metals, and check for prefixes and suffixes.
What is the difference between a cation and an anion?
+A cation is a positively charged ion, typically formed by metals, while an anion is a negatively charged ion, often formed by nonmetals.
How do I determine the charge of an ion?
+The charge of an ion is determined by the number of electrons gained or lost during the formation of the ion. Monatomic ions typically have a charge of 1- or 2- for anions and 1+ or 2+ for cations.
Why do I need to use Roman numerals for transition metals?
+Transition metals can form ions with different charges, so it’s essential to indicate the charge using Roman numerals to avoid confusion.