5 Periodic Table Tips
Unlocking the Secrets of the Periodic Table: 5 Essential Tips
The periodic table is a powerful tool used by chemists and scientists to understand the properties and behavior of elements. It’s a complex and intricate table that can seem overwhelming at first, but with the right guidance, you can unlock its secrets and become a master of chemistry. Here are five periodic table tips to help you get started:
Tip 1: Understand the Structure of the Periodic Table
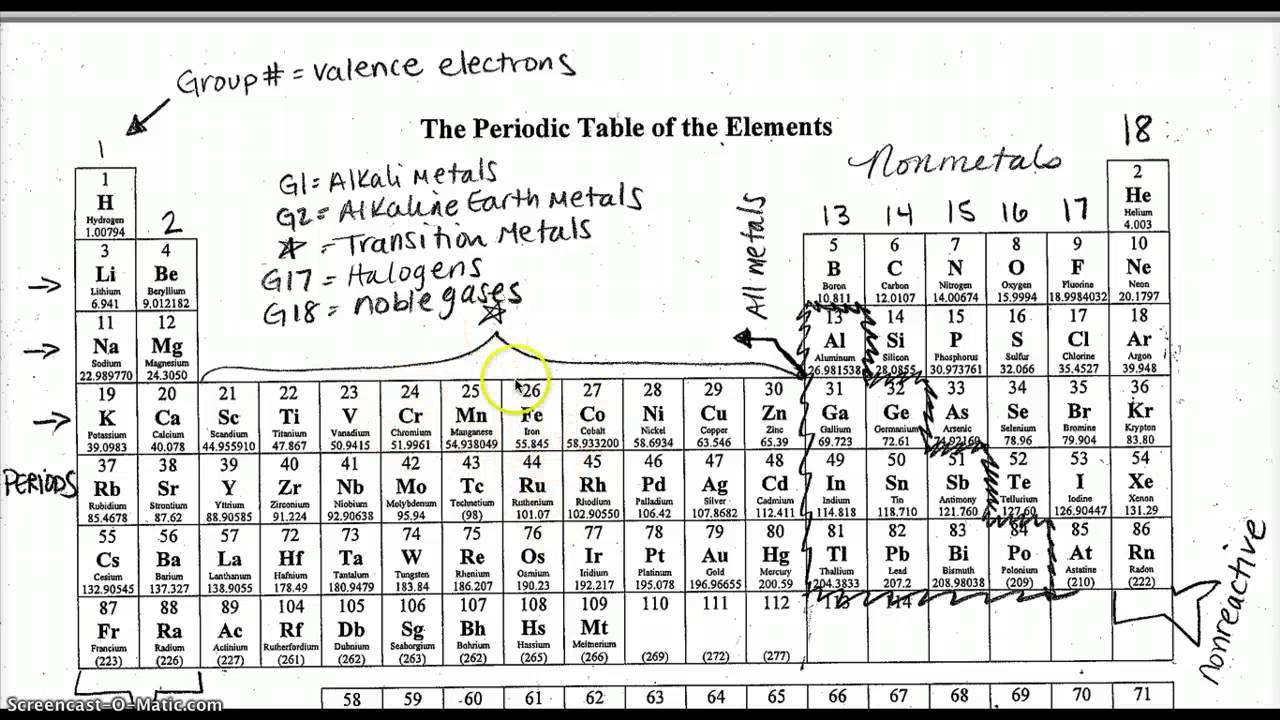
The periodic table is arranged in a logical and systematic way, with elements grouped into rows (periods) and columns (groups or families). The elements are listed in order of increasing atomic number (number of protons in the nucleus) and are grouped by their chemical properties. Rows (periods) are horizontal and columns (groups) are vertical. Understanding the structure of the periodic table is crucial to navigating and using it effectively.
Tip 2: Identify Element Blocks
The periodic table is divided into four main blocks: s-block, p-block, d-block, and f-block. Each block is characterized by the type of orbital (energy level) that the electrons occupy. The blocks are:
- s-block: elements with one or two electrons in the outermost energy level (groups 1 and 2)
- p-block: elements with three or more electrons in the outermost energy level (groups 13-18)
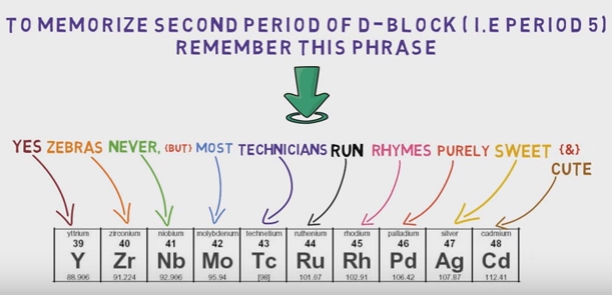
- d-block: elements with partially filled d orbitals (groups 3-12)
- f-block: elements with partially filled f orbitals (lanthanides and actinides)
📝 Note: understanding element blocks can help you identify the properties and behavior of elements and their compounds.
Tip 3: Use Periodic Trends to Predict Properties
The periodic table displays trends in element properties, such as atomic radius, electronegativity, and ionization energy. By understanding these trends, you can predict the properties of elements and their compounds. For example, elements in the same group (column) tend to have similar chemical properties, while elements in the same period (row) tend to have similar physical properties.
| Trend | Description |
|---|---|
| Atomic Radius | Increases down a group, decreases across a period |
| Electronegativity | Increases across a period, decreases down a group |
| Ionization Energy | Increases across a period, decreases down a group |
Tip 4: Identify Element Families
Element families, also known as groups, are vertical columns of elements that exhibit similar chemical properties. The most common element families are:
- Alkali Metals (group 1): highly reactive, readily lose one electron
- Alkaline Earth Metals (group 2): less reactive than alkali metals, readily lose two electrons
- Halogens (group 17): highly reactive, readily gain one electron
- Noble Gases (group 18): unreactive, full outer energy level
📝 Note: understanding element families can help you predict the chemical behavior of elements and their compounds.
Tip 5: Use the Periodic Table to Identify Element Symbols
The periodic table is a great resource for identifying element symbols. Each element has a unique one- or two-letter symbol, which is used to represent the element in chemical equations and formulas. By using the periodic table, you can quickly identify the symbol of an element and use it to write chemical equations and formulas.
In conclusion, the periodic table is a powerful tool that can help you understand the properties and behavior of elements. By following these five periodic table tips, you can unlock the secrets of the periodic table and become a master of chemistry.
What is the periodic table?
+The periodic table is a tabular arrangement of the known chemical elements, organized by their atomic number, electron configuration, and recurring chemical properties.
Why is the periodic table important?
+The periodic table is important because it allows us to understand the properties and behavior of elements, which is crucial for chemistry, physics, and other scientific fields.
How do I use the periodic table to identify element symbols?
+To use the periodic table to identify element symbols, simply find the element on the table and look at its symbol, which is usually a one- or two-letter abbreviation of the element’s name.