6 Ways to Master Naming Ionic Compounds
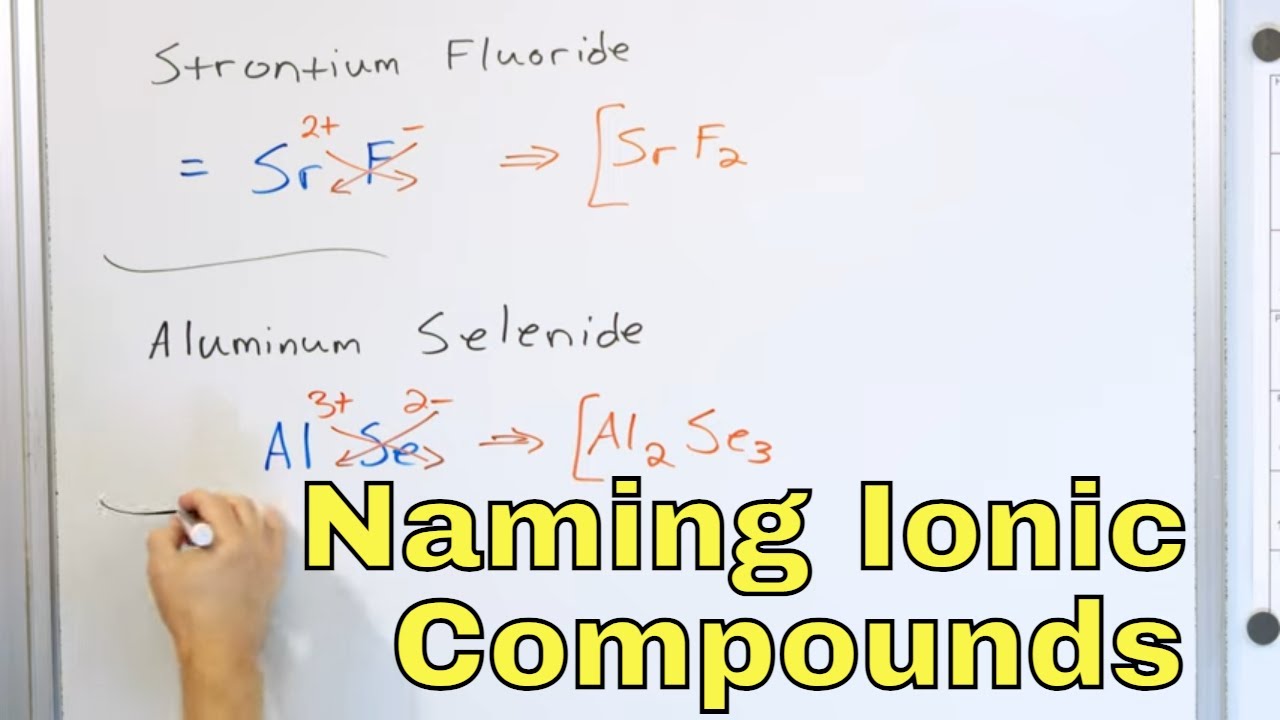
Understanding Ionic Compounds
Ionic compounds are formed when one or more electrons are transferred between atoms, resulting in a chemical bond. This process creates a molecule with a positive ion (cation) and a negative ion (anion). Mastering the naming of ionic compounds is crucial in chemistry, as it helps identify the composition and properties of these molecules. In this article, we will explore six ways to master naming ionic compounds.
1. Learn the Rules for Naming Ionic Compounds
The International Union of Pure and Applied Chemistry (IUPAC) provides a set of rules for naming ionic compounds. Familiarize yourself with these rules, which include:
- Naming the cation first, followed by the anion
- Using the suffix “-ide” for anions
- Using the suffix “-ite” and “-ate” for oxyanions
- Indicating the charge on the cation using a Roman numeral in parentheses
📝 Note: Understanding the IUPAC rules is essential for accurately naming ionic compounds.
2. Practice with Monatomic Ions
Monatomic ions are ions formed from a single atom. Practice naming compounds with monatomic ions, such as:
- Sodium chloride (NaCl)
- Calcium oxide (CaO)
- Aluminum sulfide (Al2S3)
Use the IUPAC rules to name these compounds, paying attention to the charge on the cation and the suffix used for the anion.
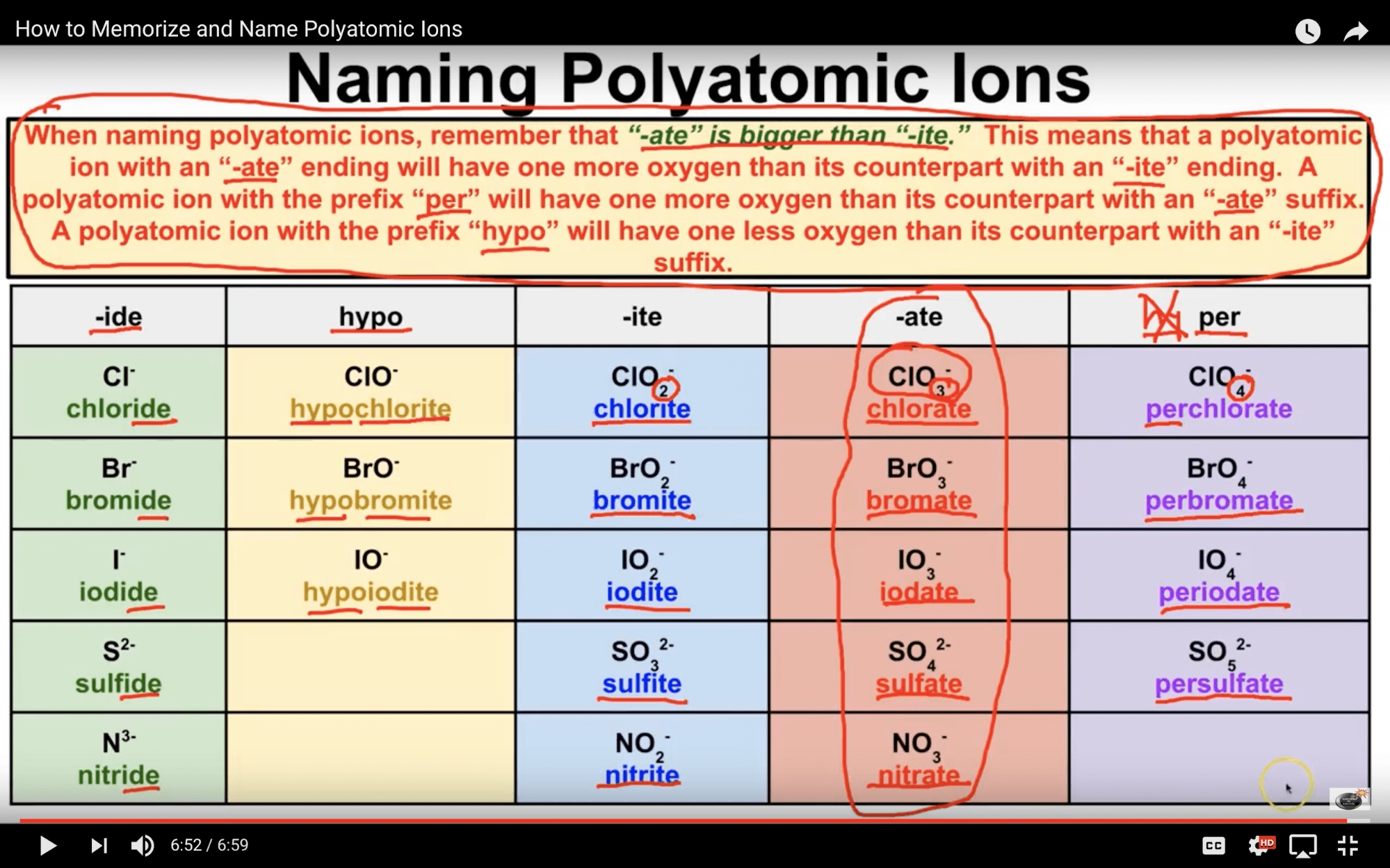
3. Master Oxyanion Naming
Oxyanions are ions that contain oxygen. Practice naming oxyanions, such as:
- Sulfate (SO42-)
- Nitrate (NO3-)
- Phosphate (PO43-)
Use the suffixes “-ite” and “-ate” to indicate the charge on the oxyanion.
💡 Note: Oxyanions can have multiple names, depending on the charge and the number of oxygen atoms.
4. Learn to Name Polyatomic Ions
Polyatomic ions are ions formed from multiple atoms. Practice naming polyatomic ions, such as:
- Ammonium (NH4+)
- Carbonate (CO32-)
- Hydroxide (OH-)
Use the IUPAC rules to name these ions, paying attention to the charge and the number of atoms.
5. Use Tables and Charts to Aid in Naming
Tables and charts can be useful tools in mastering the naming of ionic compounds. Create a table or chart with the following information:
| Cation | Anion | Compound Name |
|---|---|---|
| Na+ | Cl- | Sodium chloride |
| Ca2+ | O2- | Calcium oxide |
| Al3+ | S2- | Aluminum sulfide |
Use this table to practice naming compounds and to check your answers.
| Cation | Anion | Compound Name |
|---|---|---|
| Na+ | Cl- | Sodium chloride |
| Ca2+ | O2- | Calcium oxide |
| Al3+ | S2- | Aluminum sulfide |
6. Practice, Practice, Practice
The key to mastering the naming of ionic compounds is practice. Practice naming compounds with different cations and anions, using the IUPAC rules and tables and charts to aid in your learning.
📚 Note: The more you practice, the more comfortable you will become with naming ionic compounds.
In conclusion, mastering the naming of ionic compounds requires a combination of understanding the IUPAC rules, practicing with monatomic and polyatomic ions, using tables and charts, and practicing regularly. With these six tips, you will be well on your way to becoming proficient in naming ionic compounds.
What is the IUPAC rule for naming ionic compounds?
+The IUPAC rule for naming ionic compounds is to name the cation first, followed by the anion, using the suffix “-ide” for anions and indicating the charge on the cation using a Roman numeral in parentheses.
What is an oxyanion?
+An oxyanion is an ion that contains oxygen, such as sulfate (SO42-) and nitrate (NO3-).
How can I practice naming ionic compounds?
+You can practice naming ionic compounds by using tables and charts, practicing with monatomic and polyatomic ions, and practicing regularly.
Related Terms:
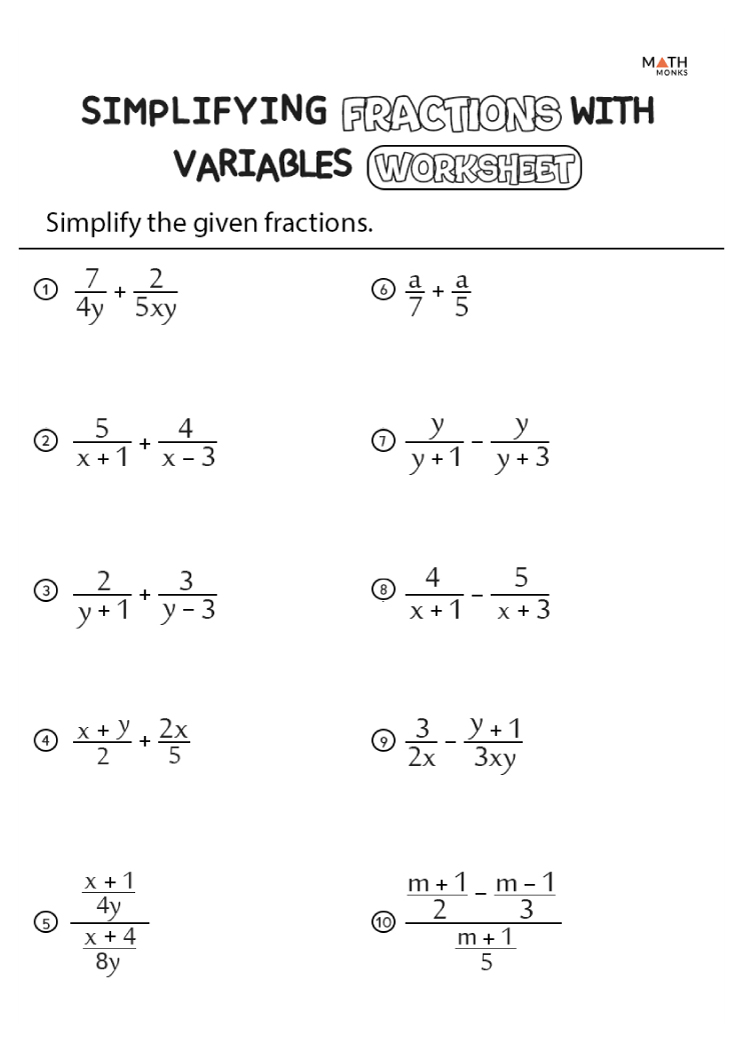
- Naming ionic compounds Worksheet pdf
- Naming Compounds Worksheet
- Naming ionic compounds worksheet POGIL
- Naming ionic compounds Worksheet 1