Naming Compounds Worksheet Answer Key Made Easy
Naming Compounds Worksheet Answer Key Made Easy
Naming compounds can be a daunting task, especially for students who are new to chemistry. However, with a few simple rules and guidelines, anyone can master the art of naming compounds. In this post, we will provide a comprehensive guide on how to name compounds, along with a worksheet answer key to help you practice and reinforce your learning.
Why is Naming Compounds Important?
Naming compounds is an essential skill in chemistry, as it allows us to communicate clearly and accurately about the substances we are working with. Without a standardized system of naming compounds, it would be impossible to distinguish between different substances, leading to confusion and potentially even danger.
The Basics of Naming Compounds
There are several types of compounds, including:
- Binary compounds: These are compounds that consist of only two elements. Examples include H2O (water) and CO2 (carbon dioxide).
- Ternary compounds: These are compounds that consist of three elements. Examples include HNO3 (nitric acid) and NaClO3 (sodium chlorate).
- Organic compounds: These are compounds that contain carbon and hydrogen atoms, often with other elements as well. Examples include methane (CH4) and ethanol (C2H5OH).
Naming Binary Compounds
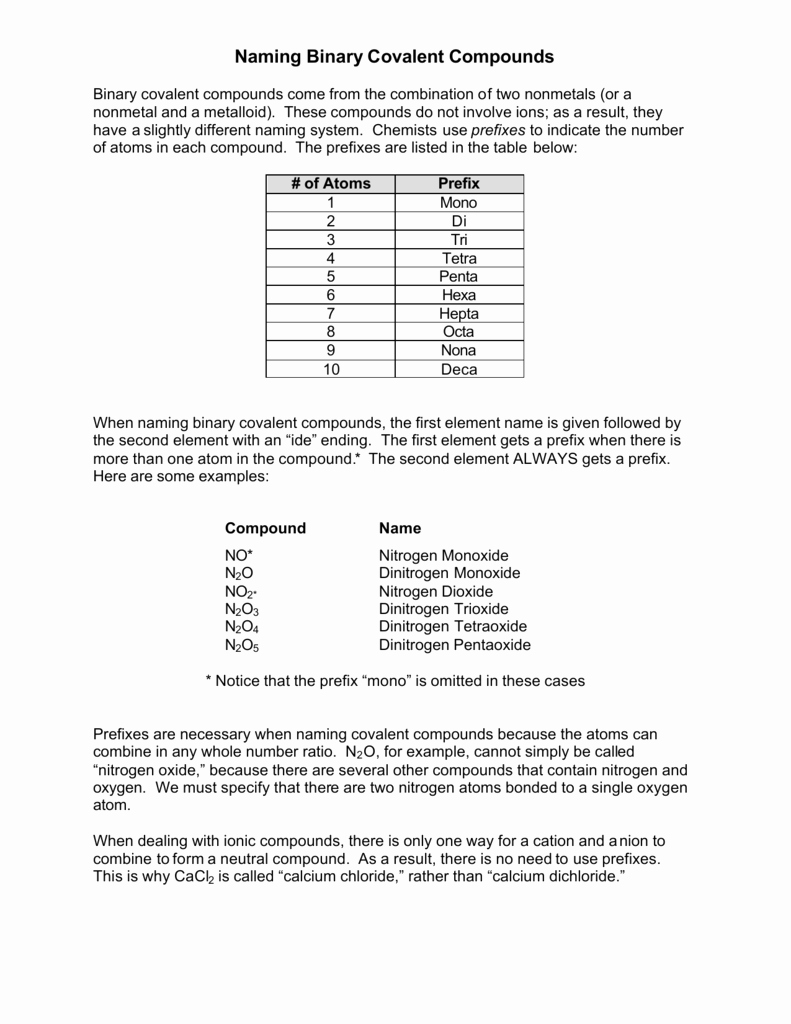
To name a binary compound, you need to know the names of the two elements it contains. The rules for naming binary compounds are as follows:
- Prefixes: Use prefixes to indicate the number of atoms of each element in the compound. The prefixes are:
- Mono- (one atom)
- Di- (two atoms)
- Tri- (three atoms)
- Tetra- (four atoms)
- Penta- (five atoms)
- Hexa- (six atoms)
- Hepta- (seven atoms)
- Octa- (eight atoms)
- Suffixes: Use suffixes to indicate the type of bond between the elements. The suffixes are:
- -ide (for a negative ion)
- -ite (for a negative ion with a smaller number of oxygen atoms)
- -ate (for a negative ion with a larger number of oxygen atoms)
- Examples:
- NaCl (sodium chloride) - sodium (Na) and chlorine (Cl)
- H2O (water) - hydrogen (H) and oxygen (O)
- CO2 (carbon dioxide) - carbon © and oxygen (O)
Naming Ternary Compounds
To name a ternary compound, you need to know the names of the three elements it contains. The rules for naming ternary compounds are as follows:
- Prefixes: Use prefixes to indicate the number of atoms of each element in the compound. The prefixes are the same as those used for binary compounds.
- Suffixes: Use suffixes to indicate the type of bond between the elements. The suffixes are the same as those used for binary compounds.
- Examples:
- HNO3 (nitric acid) - hydrogen (H), nitrogen (N), and oxygen (O)
- NaClO3 (sodium chlorate) - sodium (Na), chlorine (Cl), and oxygen (O)
Naming Organic Compounds
To name an organic compound, you need to know the names of the carbon and hydrogen atoms it contains, as well as any other elements it may contain. The rules for naming organic compounds are as follows:
- Prefixes: Use prefixes to indicate the number of carbon and hydrogen atoms in the compound. The prefixes are:
- Meth- (one carbon atom)
- Eth- (two carbon atoms)
- Prop- (three carbon atoms)
- But- (four carbon atoms)
- Suffixes: Use suffixes to indicate the type of bond between the carbon and hydrogen atoms. The suffixes are:
- -ane (for a single bond)
- -ene (for a double bond)
- -yne (for a triple bond)
- Examples:
- Methane (CH4) - one carbon atom and four hydrogen atoms
- Ethanol (C2H5OH) - two carbon atoms, five hydrogen atoms, and one oxygen atom
Worksheet Answer Key
Here are the answers to a sample worksheet on naming compounds:
- What is the name of the compound with the formula HCl? Answer: Hydrogen chloride
- What is the name of the compound with the formula CO2? Answer: Carbon dioxide
- What is the name of the compound with the formula NaClO3? Answer: Sodium chlorate
- What is the name of the compound with the formula CH4? Answer: Methane
- What is the name of the compound with the formula C2H5OH? Answer: Ethanol
📝 Note: Remember to always check the number of atoms of each element in the compound and use the correct prefixes and suffixes to name the compound correctly.
In conclusion, naming compounds is an essential skill in chemistry that requires practice and patience. By following the rules and guidelines outlined in this post, you should be able to name compounds with ease. Remember to always check your work and use the correct prefixes and suffixes to ensure accuracy.
What is the difference between a binary compound and a ternary compound?
+A binary compound is a compound that consists of only two elements, while a ternary compound is a compound that consists of three elements.
What is the purpose of using prefixes and suffixes in naming compounds?
+Prefixes are used to indicate the number of atoms of each element in the compound, while suffixes are used to indicate the type of bond between the elements.
How do I name an organic compound?
+To name an organic compound, you need to know the names of the carbon and hydrogen atoms it contains, as well as any other elements it may contain. Use prefixes to indicate the number of carbon and hydrogen atoms, and suffixes to indicate the type of bond between the carbon and hydrogen atoms.