Naming Binary Compounds Worksheet With Answers
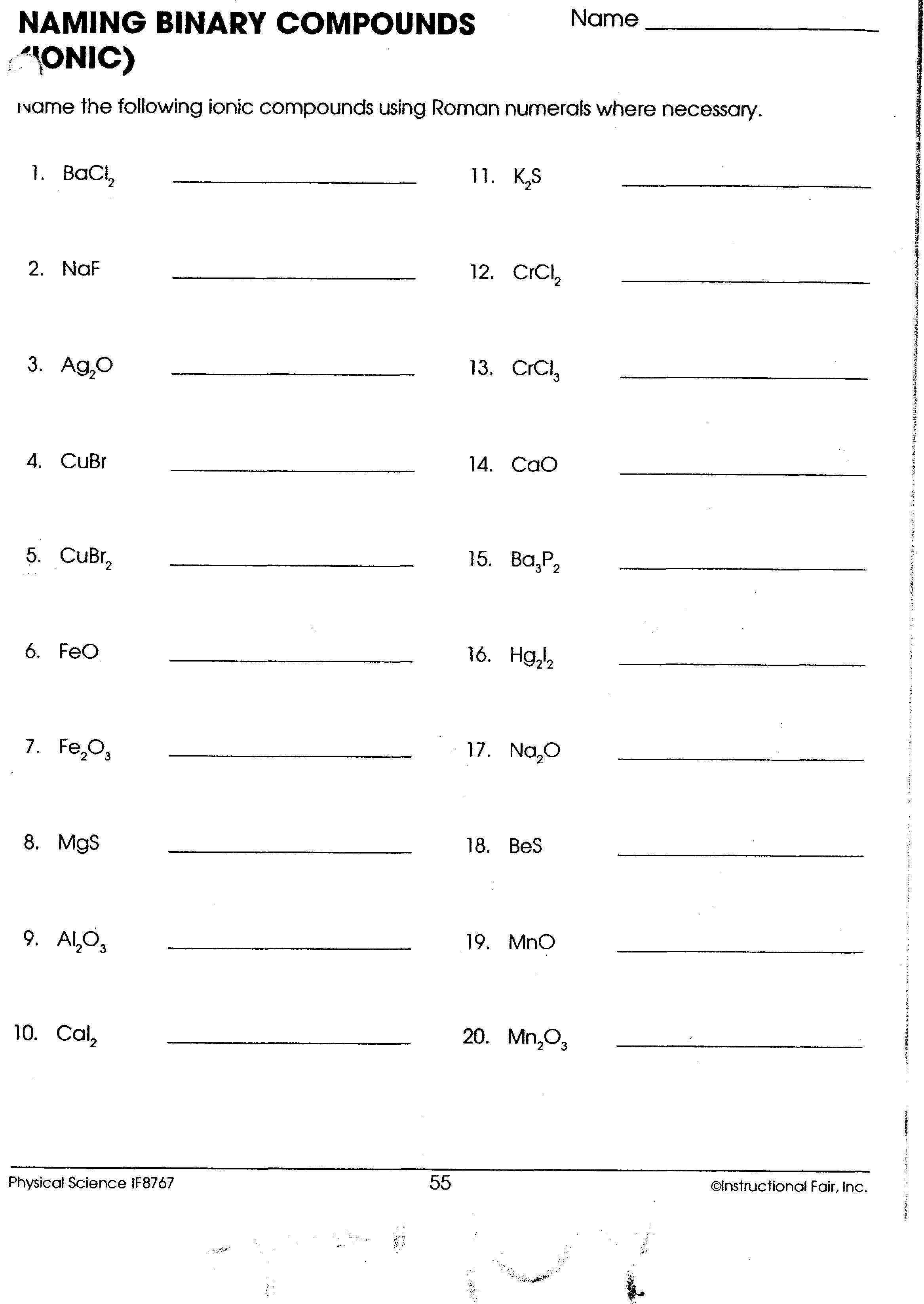
Naming Binary Compounds: A Comprehensive Guide
Binary compounds are chemical compounds that consist of two different elements. These compounds can be ionic or molecular, and their names are derived from the elements that make them up. In this article, we will explore the rules for naming binary compounds, provide examples, and offer a worksheet with answers to help you practice.
Types of Binary Compounds
There are two main types of binary compounds: ionic and molecular.
- Ionic compounds: These compounds are formed when one or more electrons are transferred between atoms, resulting in the formation of ions. The ions are then attracted to each other and form a compound. Examples of ionic compounds include sodium chloride (NaCl) and calcium carbonate (CaCO3).
- Molecular compounds: These compounds are formed when two or more atoms share one or more pairs of electrons. This sharing of electrons results in a covalent bond. Examples of molecular compounds include water (H2O) and carbon dioxide (CO2).
Naming Binary Compounds
The rules for naming binary compounds are different for ionic and molecular compounds.
Ionic Compounds
When naming ionic compounds, the name of the cation (the positively charged ion) is written first, followed by the name of the anion (the negatively charged ion). The suffix “-ide” is added to the root of the anion.
- Examples:
- Sodium chloride (NaCl) - sodium is the cation, and chloride is the anion
- Calcium carbonate (CaCO3) - calcium is the cation, and carbonate is the anion
- Iron(III) oxide (Fe2O3) - iron is the cation, and oxide is the anion
Molecular Compounds
When naming molecular compounds, the prefix “mono-” is used to indicate one atom of an element, “di-” is used to indicate two atoms, “tri-” is used to indicate three atoms, and so on. The suffix “-ide” is added to the root of the second element.
- Examples:
- Water (H2O) - two hydrogen atoms and one oxygen atom
- Carbon dioxide (CO2) - one carbon atom and two oxygen atoms
- Ammonia (NH3) - one nitrogen atom and three hydrogen atoms
Worksheet: Naming Binary Compounds
Here is a worksheet with 10 questions to help you practice naming binary compounds. The answers are provided below.
Worksheet:
- What is the name of the compound with the formula Na2O?
- What is the name of the compound with the formula CO2?
- What is the name of the compound with the formula FeCl3?
- What is the name of the compound with the formula H2S?
- What is the name of the compound with the formula CaF2?
- What is the name of the compound with the formula NH3?
- What is the name of the compound with the formula Al2O3?
- What is the name of the compound with the formula SiO2?
- What is the name of the compound with the formula KI?
- What is the name of the compound with the formula MgCl2?
Answers:
- Sodium oxide
- Carbon dioxide
- Iron(III) chloride
- Hydrogen sulfide
- Calcium fluoride
- Ammonia
- Aluminum oxide
- Silicon dioxide
- Potassium iodide
- Magnesium chloride
📝 Note: Remember to use the correct suffixes and prefixes when naming binary compounds.
In conclusion, naming binary compounds requires a good understanding of the rules for ionic and molecular compounds. With practice and patience, you can become proficient in naming these compounds. Remember to use the correct suffixes and prefixes, and to write the name of the cation before the name of the anion for ionic compounds. For molecular compounds, use the prefix “mono-” to indicate one atom of an element, and the suffix “-ide” to indicate the second element.
What is the difference between ionic and molecular compounds?
+Ionic compounds are formed when one or more electrons are transferred between atoms, resulting in the formation of ions. Molecular compounds are formed when two or more atoms share one or more pairs of electrons.
How do you name ionic compounds?
+The name of the cation is written first, followed by the name of the anion. The suffix “-ide” is added to the root of the anion.
What is the prefix used to indicate one atom of an element in molecular compounds?
+The prefix “mono-” is used to indicate one atom of an element.
Related Terms:
- Naming Binary Compounds Worksheet pdf
- Naming binary ionic compounds Worksheet