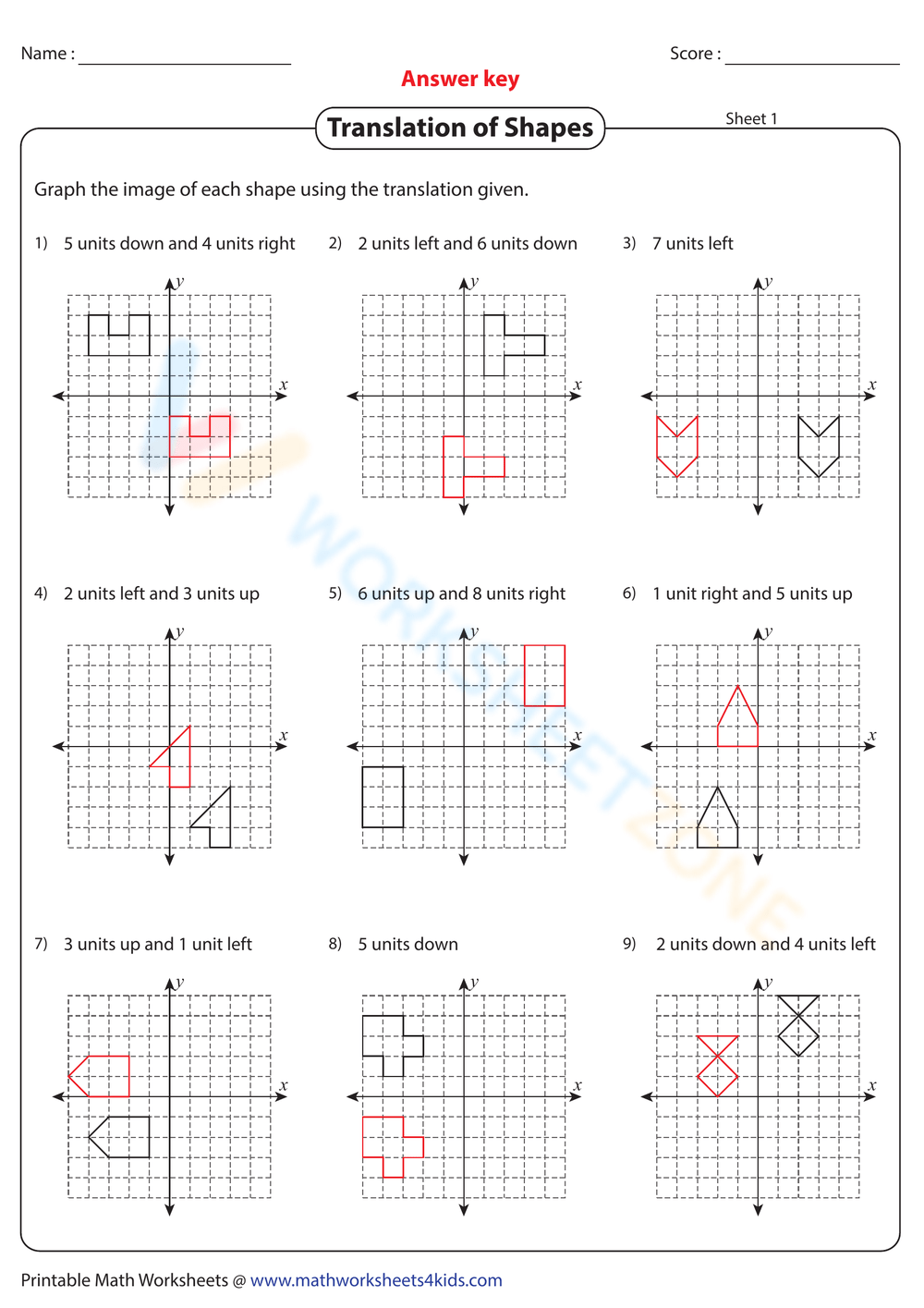
Binary Compounds Naming Worksheet Answers Guide
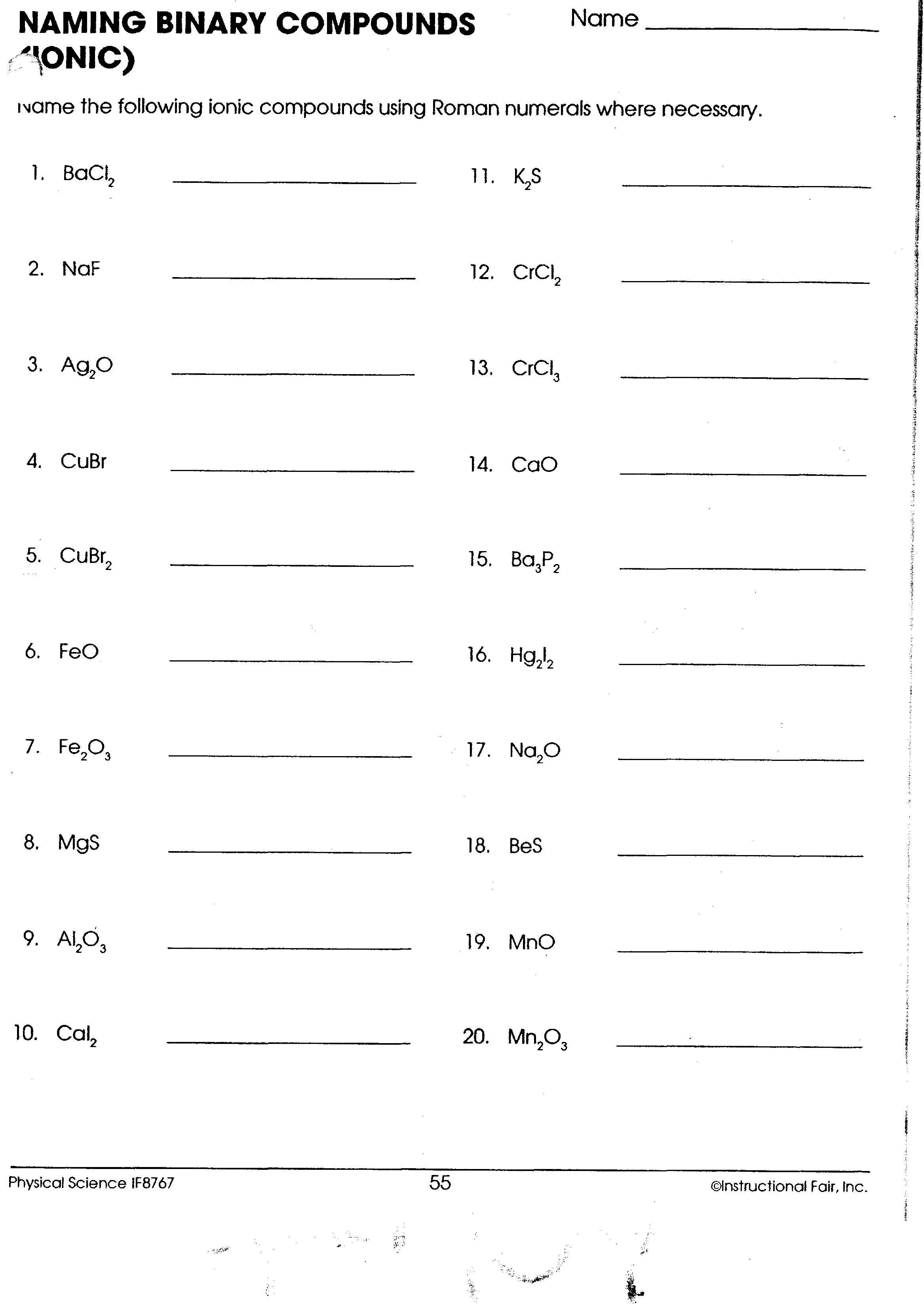
Understanding Binary Compounds
Binary compounds are chemical compounds that consist of exactly two different elements. These elements can be metals, nonmetals, or a combination of both. The process of naming these compounds is straightforward and based on a set of rules established by the International Union of Pure and Applied Chemistry (IUPAC).
Rules for Naming Binary Compounds
The rules for naming binary compounds differ slightly depending on whether the compound consists of two nonmetals, a metal and a nonmetal, or a polyatomic ion and another element.
Compounds Consisting of Two Nonmetals
- When naming compounds consisting of two nonmetals, the prefix indicating the number of each atom is included. The prefix “mono-” is usually omitted for the first element.
- The name of the second nonmetal is modified to end in “-ide.”
Compounds Consisting of a Metal and a Nonmetal
- For compounds consisting of a metal and a nonmetal, the name of the metal comes first, followed by the base name of the nonmetal modified to end in “-ide.”
- If the metal can form ions with different charges, a Roman numeral in parentheses is included after the name of the metal to indicate the charge.
Compounds Consisting of a Polyatomic Ion and Another Element
- Polyatomic ions are groups of atoms that behave as a single ion. When naming compounds consisting of a polyatomic ion and another element, the name of the polyatomic ion is used.
- If the polyatomic ion is combined with a metal, the name of the metal is followed by the name of the polyatomic ion.
Examples of Naming Binary Compounds
- CO2: Carbon dioxide (prefixes are used to indicate the number of each atom)
- NaCl: Sodium chloride (the metal’s name comes first, followed by the nonmetal’s name modified to end in “-ide”)
- CaCO3: Calcium carbonate (the metal’s name comes first, followed by the polyatomic ion’s name)
- NH3: Ammonia (the name of the polyatomic ion is used)
Worksheets for Practice
Here are some examples of binary compounds to practice naming:
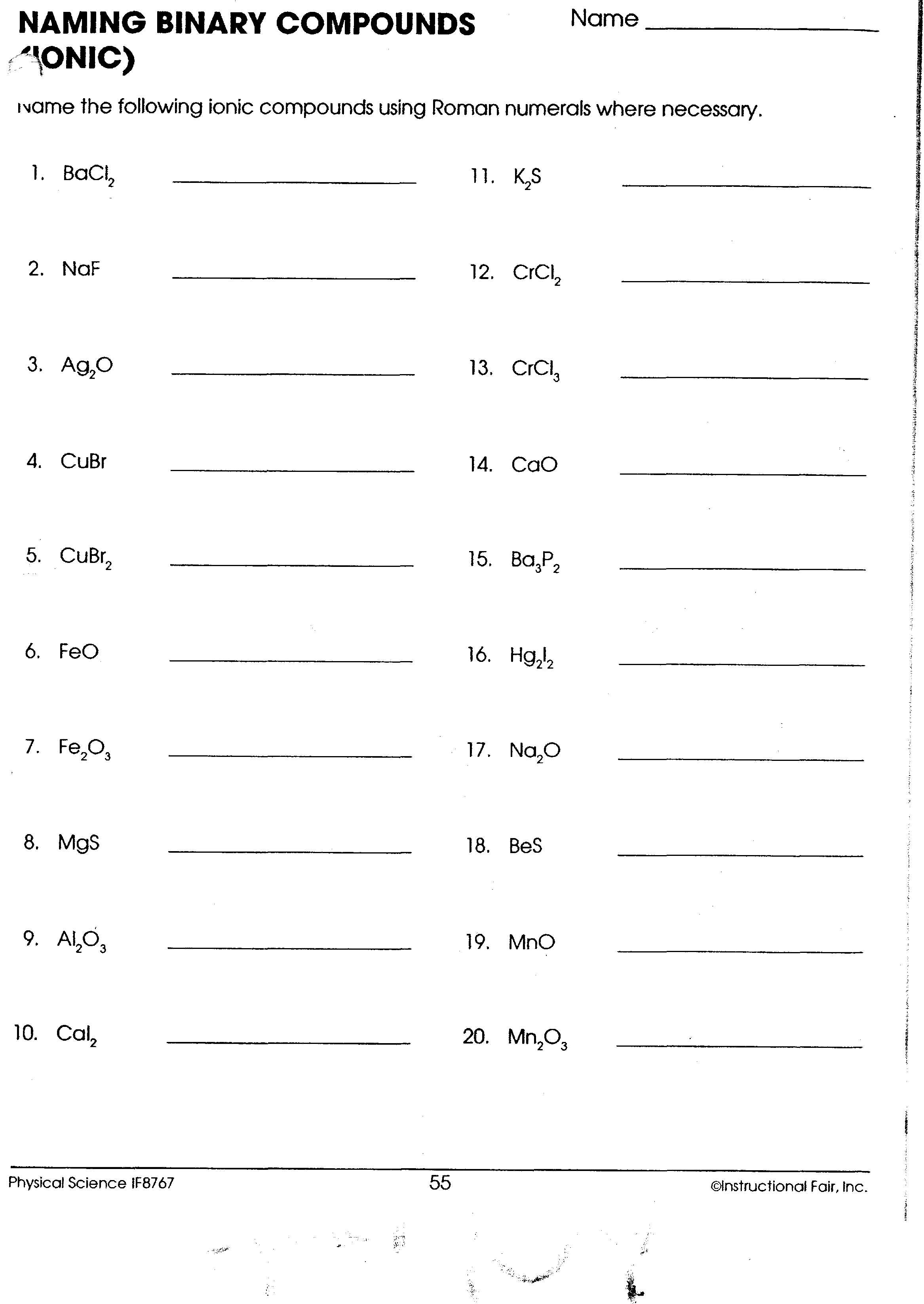
| Formula | Name |
|---|---|
| CO2 | |
| NaCl | |
| CaCO3 | |
| NH3 |
Answers:
| Formula | Name |
|---|---|
| CO2 | Carbon dioxide |
| NaCl | Sodium chloride |
| CaCO3 | Calcium carbonate |
| NH3 | Ammonia |
Conclusion
Naming binary compounds requires a basic understanding of the rules established by the IUPAC. By practicing with different types of compounds, you can become proficient in naming binary compounds.
What is the main rule for naming binary compounds consisting of two nonmetals?
+The main rule is to include the prefix indicating the number of each atom and to modify the name of the second nonmetal to end in “-ide.”
How do you name compounds consisting of a metal and a nonmetal?
+The name of the metal comes first, followed by the base name of the nonmetal modified to end in “-ide.” If the metal can form ions with different charges, a Roman numeral in parentheses is included after the name of the metal.
What is the rule for naming compounds consisting of a polyatomic ion and another element?
+The name of the polyatomic ion is used. If the polyatomic ion is combined with a metal, the name of the metal is followed by the name of the polyatomic ion.
Related Terms:
- Naming Binary Compounds Worksheet pdf
- Naming binary ionic compounds Worksheet
- Naming ionic compounds Worksheet one