5 Ways to Master Naming Covalent Compounds
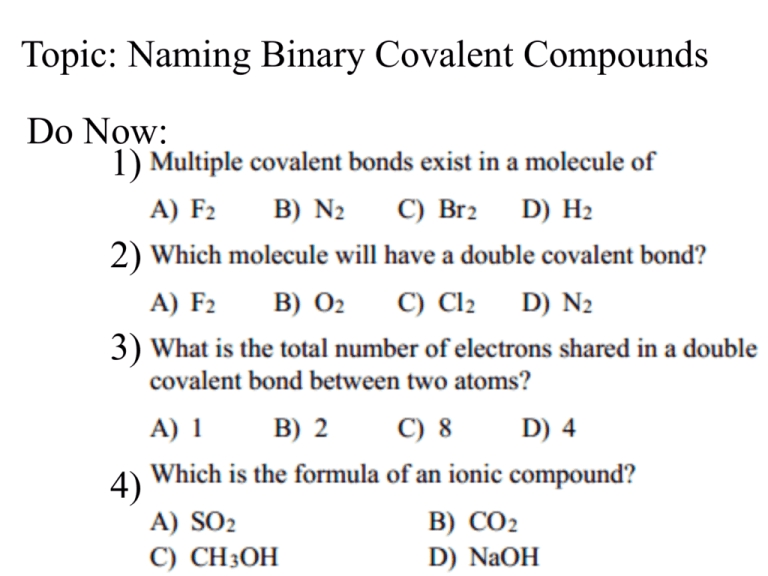
Understanding Covalent Compounds and Their Naming
Covalent compounds are a class of chemical compounds where the atoms share electrons to form a molecule. These compounds are typically found in molecules that consist of nonmetal elements, and they are very common in everyday life. One of the most important aspects of studying covalent compounds is learning how to name them correctly. In this article, we will explore the world of covalent compounds and provide you with five ways to master their naming.
What Are Covalent Compounds?
Before we dive into the naming of covalent compounds, let’s take a brief look at what they are. Covalent compounds are formed when two or more nonmetal atoms share electrons to form a molecule. This sharing of electrons leads to the formation of a chemical bond between the atoms. Covalent compounds can be either polar or nonpolar, depending on the difference in electronegativity between the atoms.
Why Is Naming Covalent Compounds Important?
Naming covalent compounds is crucial in chemistry because it allows us to communicate effectively about the compounds we are working with. Without a standardized system of naming, it would be difficult to identify and discuss the various compounds. Furthermore, naming covalent compounds helps us to identify their properties and behaviors, which is essential in fields such as medicine, materials science, and environmental science.
5 Ways to Master Naming Covalent Compounds
1. Learn the Prefixes and Suffixes
The first step to mastering the naming of covalent compounds is to learn the prefixes and suffixes used in their naming. The prefixes used in naming covalent compounds indicate the number of atoms of each element present in the compound. The most common prefixes are:
- Mono- (1 atom)
- Di- (2 atoms)
- Tri- (3 atoms)
- Tetra- (4 atoms)
- Penta- (5 atoms)
The suffixes used in naming covalent compounds indicate the type of bond present in the compound. The most common suffixes are:
- -ide (for compounds with a single bond)
- -ite (for compounds with a double bond)
- -ate (for compounds with a triple bond)
2. Understand the Rules for Naming Binary Covalent Compounds
Binary covalent compounds are compounds that consist of two different nonmetal elements. The naming of binary covalent compounds follows a specific set of rules:
- The first element in the compound is named using its full name.
- The second element in the compound is named using its root name with the suffix -ide.
- If the first element is a single atom, the prefix mono- is not used.
- If the first element is a multiple atom, the prefix is used.
Example: CO (carbon monoxide)
3. Learn to Name Compounds with Multiple Bonds
Compounds with multiple bonds are named using the same rules as binary covalent compounds, with a few additional rules:
- If the compound has a double bond, the suffix -ite is used.
- If the compound has a triple bond, the suffix -ate is used.
- The prefixes are used to indicate the number of atoms of each element present in the compound.
Example: CO2 (carbon dioxide)
4. Practice Naming Compounds with Multiple Elements
Once you have mastered the naming of binary covalent compounds, you can move on to compounds with multiple elements. The naming of these compounds follows the same rules as binary covalent compounds, with a few additional rules:
- The elements are named in the order they appear in the compound.
- The prefixes are used to indicate the number of atoms of each element present in the compound.
- The suffixes are used to indicate the type of bond present in the compound.
Example: CH4 (methane)
5. Use Online Resources to Practice
The final step to mastering the naming of covalent compounds is to practice, practice, practice! There are many online resources available that can help you practice naming covalent compounds, including:
- Online quizzes and games
- Interactive tutorials and videos
- Practice exercises and worksheets
By following these five steps, you can master the naming of covalent compounds and become a pro at identifying and naming these important chemical compounds.
🤔 Note: The key to mastering the naming of covalent compounds is to practice, practice, practice! Use online resources to practice naming compounds, and you will become a pro in no time.
Without a doubt, mastering the naming of covalent compounds is an essential skill for any student of chemistry. By following the five steps outlined above, you can become proficient in naming covalent compounds and take your knowledge of chemistry to the next level.
What is a covalent compound?
+A covalent compound is a chemical compound where the atoms share electrons to form a molecule.
Why is naming covalent compounds important?
+Naming covalent compounds is important because it allows us to communicate effectively about the compounds we are working with and to identify their properties and behaviors.
What are the prefixes used in naming covalent compounds?
+The prefixes used in naming covalent compounds are: mono- (1 atom), di- (2 atoms), tri- (3 atoms), tetra- (4 atoms), and penta- (5 atoms).