Common Isotopes Worksheet Answers and Practice Problems
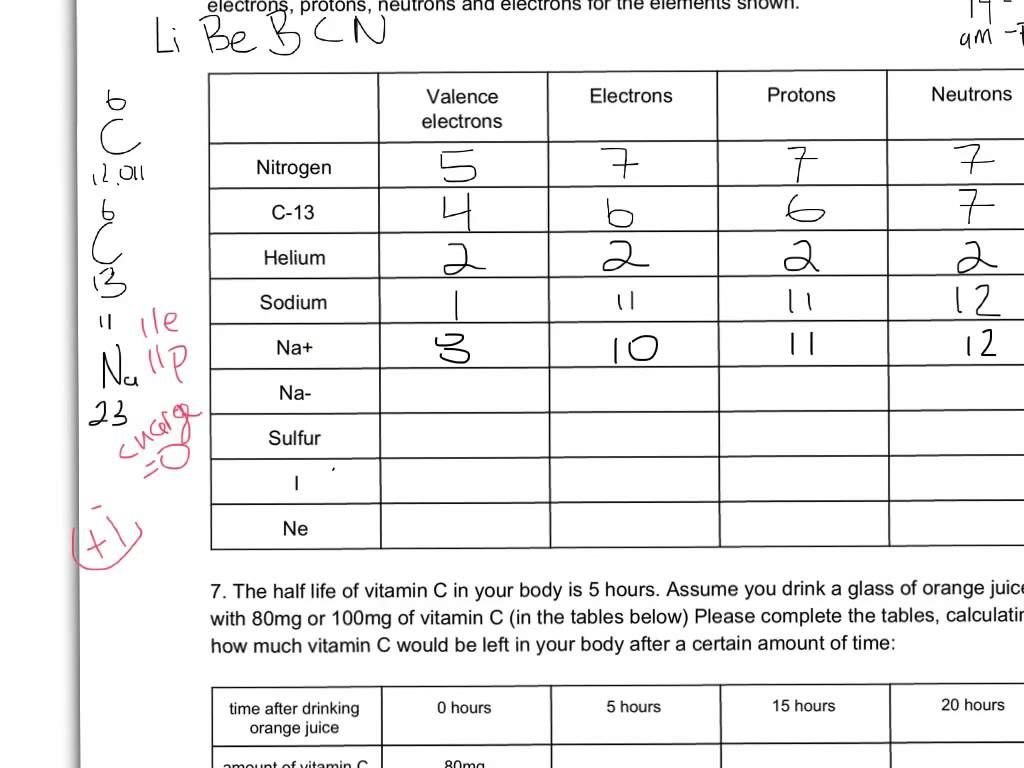
Understanding Isotopes and Their Significance
Isotopes are atoms of the same element that have the same number of protons in their atomic nuclei but differ in the number of neutrons. This variation in neutron number affects the atomic mass of the isotopes, leading to different physical and chemical properties. In this worksheet, we will explore the concept of isotopes, their types, and practice problems to reinforce understanding.
Types of Isotopes
Isotopes can be categorized into two main types: stable and radioactive. Stable isotopes do not undergo radioactive decay, whereas radioactive isotopes are unstable and decay into other elements over time.
📝 Note: The most common isotope of an element is usually the stable one, but this is not always the case.
Identifying Isotopes
To identify an isotope, you need to know the atomic number (number of protons) and the mass number (total number of protons and neutrons) of the element. The atomic number defines the element, while the mass number helps distinguish between isotopes of the same element.
Example 1: Identifying an Isotope
An atom has an atomic number of 6 and a mass number of 14. What is the element, and how many neutrons does it have?
- Atomic number = 6 ( Carbon )
- Mass number = 14
- Number of protons = 6
- Number of neutrons = Mass number - Atomic number = 14 - 6 = 8
The element is Carbon-14, and it has 8 neutrons.
Example 2: Identifying an Isotope
An atom has an atomic number of 8 and a mass number of 18. What is the element, and how many neutrons does it have?
- Atomic number = 8 ( Oxygen )
- Mass number = 18
- Number of protons = 8
- Number of neutrons = Mass number - Atomic number = 18 - 8 = 10
The element is Oxygen-18, and it has 10 neutrons.
Practice Problems
- An atom has an atomic number of 12 and a mass number of 24. What is the element, and how many neutrons does it have?
- Answer: The element is Magnesium-24, and it has 12 neutrons.
- Atomic number = 12 ( Magnesium )
- Mass number = 24
- Number of protons = 12
- Number of neutrons = Mass number - Atomic number = 24 - 12 = 12
- An atom has an atomic number of 20 and a mass number of 40. What is the element, and how many neutrons does it have?
- Answer: The element is Calcium-40, and it has 20 neutrons.
- Atomic number = 20 ( Calcium )
- Mass number = 40
- Number of protons = 20
- Number of neutrons = Mass number - Atomic number = 40 - 20 = 20
Additional Practice Problems

| Atomic Number | Mass Number | Element | Number of Neutrons |
|---|---|---|---|
| 1 | 2 | Hydrogen | ? |
| 6 | 12 | Carbon | ? |
| 8 | 16 | Oxygen | ? |
| 15 | 31 | Phosphorus | ? |
Answers:
| Atomic Number | Mass Number | Element | Number of Neutrons |
|---|---|---|---|
| 1 | 2 | Hydrogen | 1 |
| 6 | 12 | Carbon | 6 |
| 8 | 16 | Oxygen | 8 |
| 15 | 31 | Phosphorus | 16 |
📝 Note: Remember to subtract the atomic number from the mass number to find the number of neutrons.
Without understanding the types of isotopes and how to identify them, it’s challenging to grasp many concepts in chemistry and physics. By practicing these problems, you’ll become more comfortable with the concept of isotopes and be better prepared to tackle more complex topics in the future.
What is the main difference between isotopes?
+The main difference between isotopes is the number of neutrons in their atomic nuclei. This variation in neutron number affects the atomic mass of the isotopes, leading to different physical and chemical properties.
How do you identify an isotope?
+To identify an isotope, you need to know the atomic number (number of protons) and the mass number (total number of protons and neutrons) of the element.
What is the significance of understanding isotopes?
+Understanding isotopes is crucial in many fields, including chemistry, physics, and geology. It helps in understanding the structure and properties of elements, which is essential for various applications in science and technology.
Related Terms:
- Most Common Isotope Worksheet 1
- Isotope Worksheet pdf
- Isotope Practice Worksheet answers PDF