Mastering Monatomic Ions with a Simple Worksheet Guide
Understanding Monatomic Ions: A Comprehensive Guide
Monatomic ions are atoms that have gained or lost electrons to form ions with a single charge. They are a fundamental concept in chemistry and are used to describe the properties and behavior of ions in various chemical reactions. In this guide, we will explore the world of monatomic ions, their formation, and provide a simple worksheet to help you master this concept.
Formation of Monatomic Ions
Monatomic ions are formed when an atom gains or loses electrons to achieve a full outer energy level. This process is known as ionization. When an atom gains electrons, it becomes a negatively charged ion, known as an anion. On the other hand, when an atom loses electrons, it becomes a positively charged ion, known as a cation.
For example, when a sodium atom loses an electron, it becomes a positively charged sodium ion (Na+). Similarly, when a chlorine atom gains an electron, it becomes a negatively charged chloride ion (Cl-).
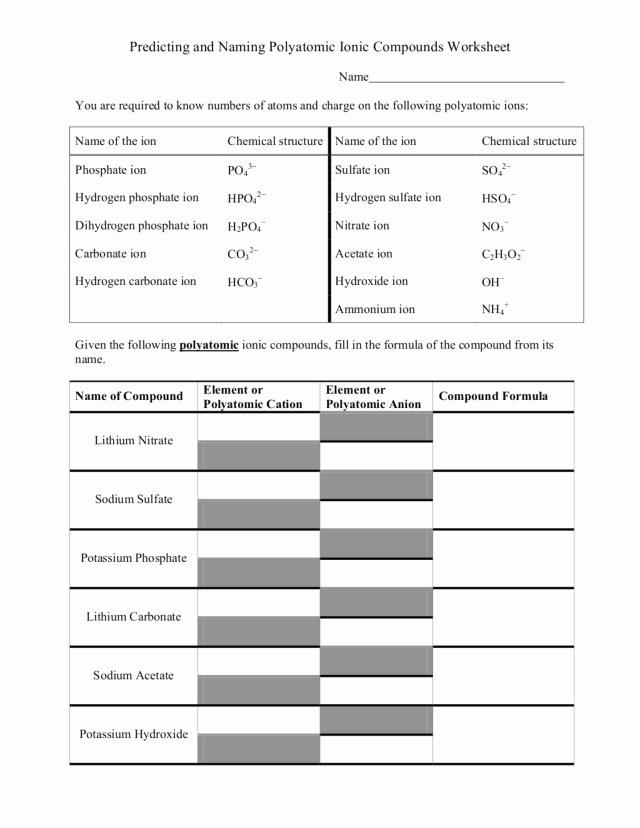
Worksheet: Identifying Monatomic Ions
To help you practice identifying monatomic ions, we have created a simple worksheet below. Please note that the answers are provided at the end of this guide.
| Atom | Ion | Charge |
|---|---|---|
| Sodium (Na) | ? | ? |
| Chlorine (Cl) | ? | ? |
| Calcium (Ca) | ? | ? |
| Oxygen (O) | ? | ? |
Rules for Writing Monatomic Ion Symbols
To write the symbol of a monatomic ion, you need to follow these simple rules:
- Cations: Write the symbol of the atom, followed by a plus sign (+) to indicate the positive charge. For example, Na+ for sodium ion.
- Anions: Write the symbol of the atom, followed by a minus sign (-) to indicate the negative charge. For example, Cl- for chloride ion.
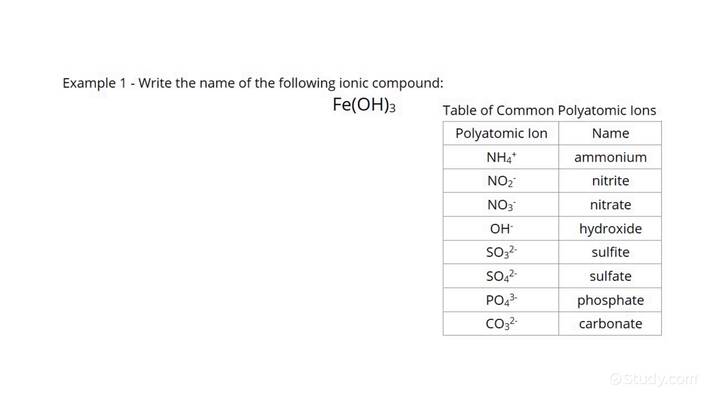
Common Monatomic Ions
Here are some common monatomic ions that you should know:
- Alkali metals: Li+ (lithium), Na+ (sodium), K+ (potassium)
- Halogens: F- (fluoride), Cl- (chloride), Br- (bromide), I- (iodide)
- Noble gases: He (helium), Ne (neon), Ar (argon), Kr (krypton), Xe (xenon)
🔥 Note: Noble gases do not typically form ions, but they can react with other elements to form compounds.
Conclusion
Mastering monatomic ions is an essential skill in chemistry. By understanding how to form and write the symbols of monatomic ions, you will be able to describe the properties and behavior of ions in various chemical reactions. Practice the worksheet provided above to reinforce your understanding of this concept.
What is a monatomic ion?
+A monatomic ion is an atom that has gained or lost electrons to form an ion with a single charge.
How are monatomic ions formed?
+Monatomic ions are formed when an atom gains or loses electrons to achieve a full outer energy level.
What is the difference between a cation and an anion?
+A cation is a positively charged ion, while an anion is a negatively charged ion.
Answers to the worksheet:
| Atom | Ion | Charge |
|---|---|---|
| Sodium (Na) | Na+ | +1 |
| Chlorine (Cl) | Cl- | -1 |
| Calcium (Ca) | Ca2+ | +2 |
| Oxygen (O) | O2- | -2 |