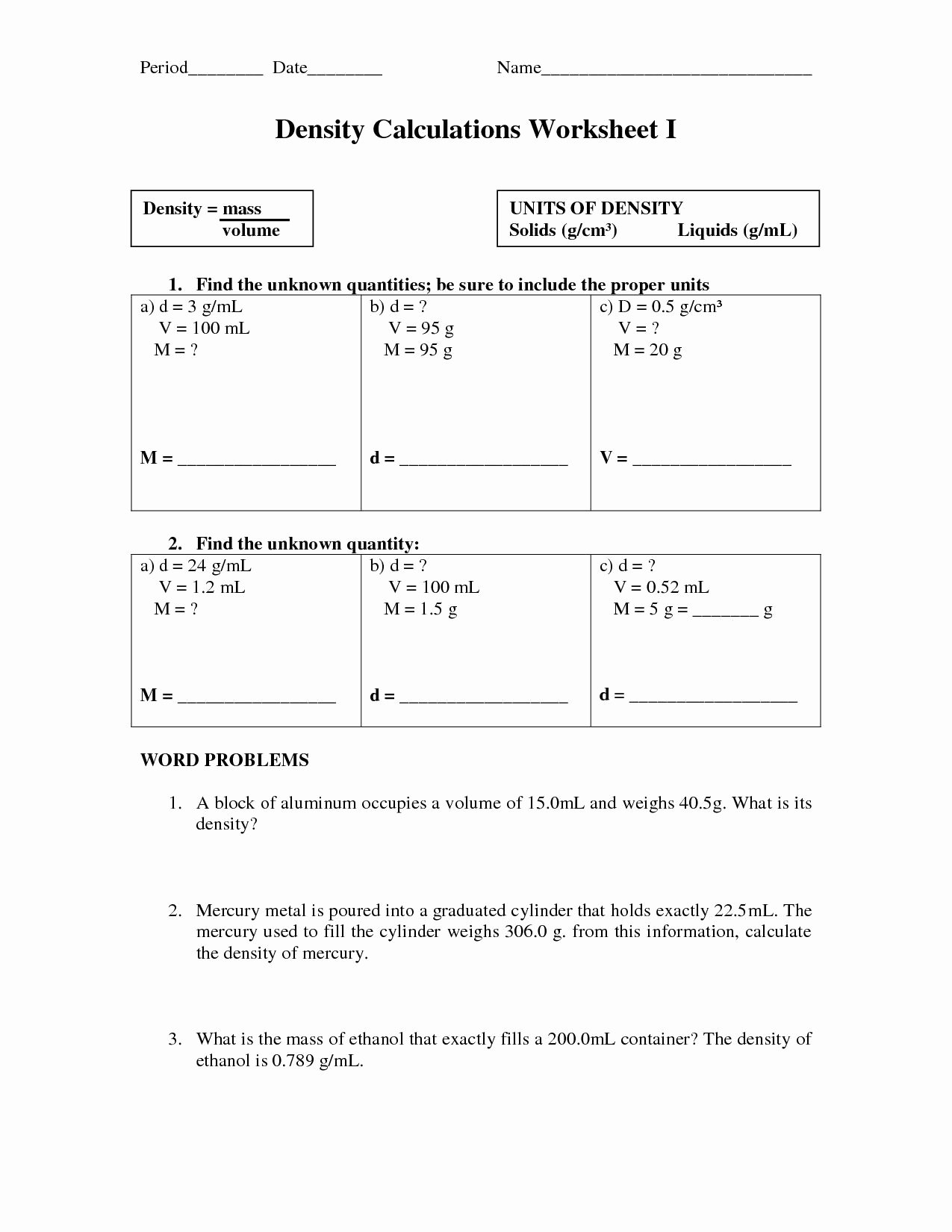
Molecular Geometry Practice Worksheet

Understanding Molecular Geometry
Molecular geometry is the study of the three-dimensional arrangement of atoms in a molecule. It is a crucial concept in chemistry, as it helps us understand the shape and structure of molecules, which in turn affects their physical and chemical properties. In this worksheet, we will practice determining the molecular geometry of various molecules using the VSEPR (Valence Shell Electron Pair Repulsion) theory.
VSEPR Theory Basics
The VSEPR theory states that electron pairs in the valence shell of an atom repel each other and arrange themselves to minimize their mutual repulsion. The resulting arrangement of electron pairs determines the molecular geometry.
Key Points to Remember:
- Electron pairs: Include both bonding pairs (shared pairs) and lone pairs (unshared pairs).
- Central atom: The atom that is bonded to multiple other atoms.
- Bonding pairs: Shared pairs of electrons between two atoms.
- Lone pairs: Unshared pairs of electrons on a single atom.
- Steric number: The total number of electron pairs (bonding and lone) on the central atom.
Determining Molecular Geometry
To determine the molecular geometry of a molecule, follow these steps:
- Draw the Lewis structure: Draw the Lewis structure of the molecule, including all bonding and lone pairs.
- Identify the central atom: Identify the central atom, which is usually the atom with the lowest group number.
- Count the electron pairs: Count the total number of electron pairs (bonding and lone) on the central atom.
- Determine the steric number: Determine the steric number, which is the total number of electron pairs on the central atom.
- Apply the VSEPR theory: Apply the VSEPR theory to determine the molecular geometry based on the steric number.
VSEPR Theory Geometry Chart:
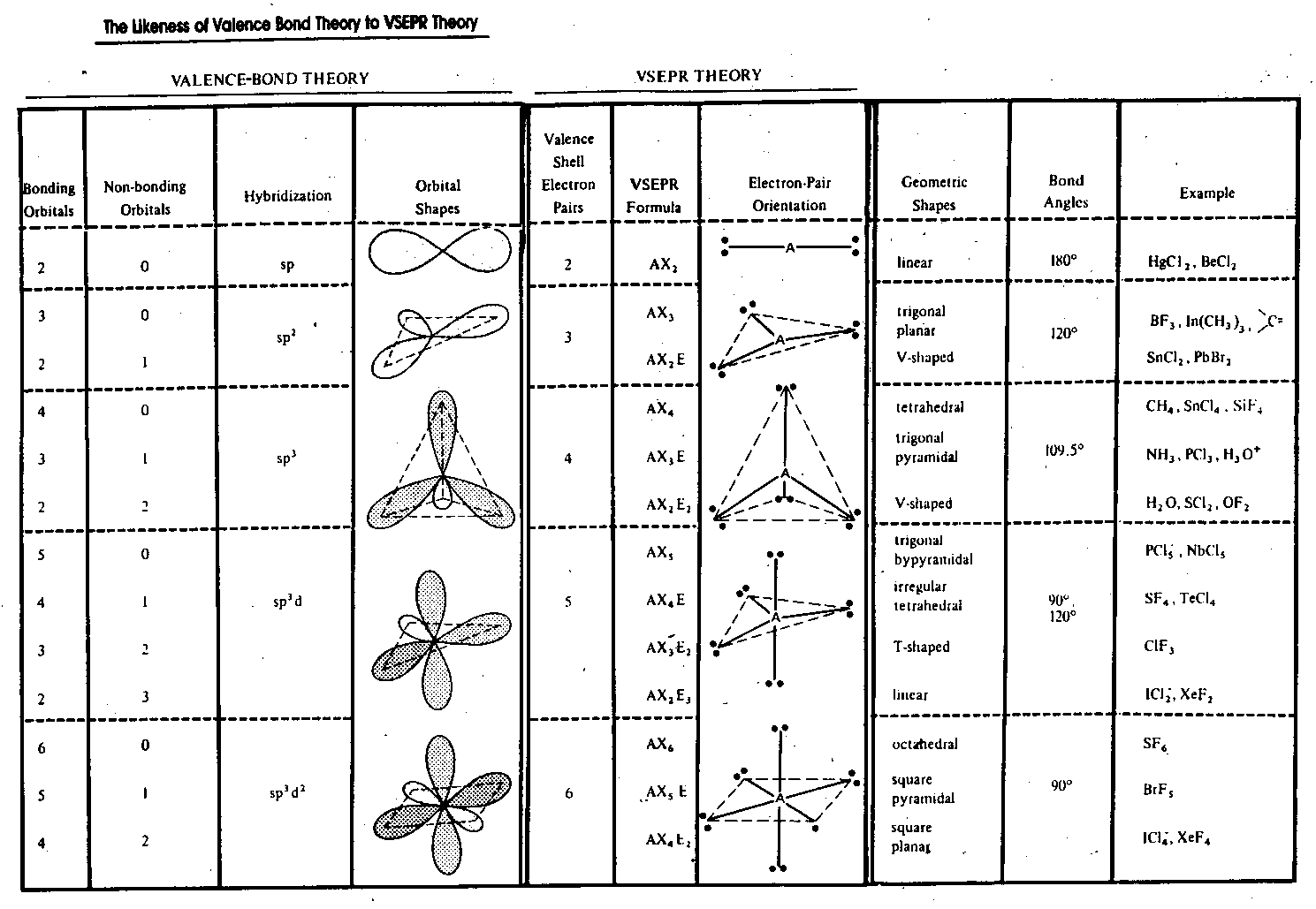
| Steric Number | Electron Pair Arrangement | Molecular Geometry |
|---|---|---|
| 2 | Linear | Linear |
| 3 | Trigonal Planar | Trigonal Planar |
| 4 | Tetrahedral | Tetrahedral |
| 5 | Trigonal Bipyramidal | Trigonal Bipyramidal |
| 6 | Octahedral | Octahedral |
Practice Problems
Problem 1: Determine the molecular geometry of CO2 (carbon dioxide).
Solution:
- Draw the Lewis structure: CO2 has a central carbon atom bonded to two oxygen atoms.
- Identify the central atom: The central atom is carbon.
- Count the electron pairs: Carbon has 4 electron pairs (2 bonding pairs and 2 lone pairs).
- Determine the steric number: The steric number is 4.
- Apply the VSEPR theory: With a steric number of 4, the electron pair arrangement is tetrahedral, and the molecular geometry is linear.
Answer: Linear
Problem 2: Determine the molecular geometry of NH3 (ammonia).
Solution:
- Draw the Lewis structure: NH3 has a central nitrogen atom bonded to three hydrogen atoms.
- Identify the central atom: The central atom is nitrogen.
- Count the electron pairs: Nitrogen has 4 electron pairs (3 bonding pairs and 1 lone pair).
- Determine the steric number: The steric number is 4.
- Apply the VSEPR theory: With a steric number of 4, the electron pair arrangement is tetrahedral, and the molecular geometry is trigonal pyramidal.
Answer: Trigonal Pyramidal
Problem 3: Determine the molecular geometry of H2O (water).
Solution:
- Draw the Lewis structure: H2O has a central oxygen atom bonded to two hydrogen atoms.
- Identify the central atom: The central atom is oxygen.
- Count the electron pairs: Oxygen has 4 electron pairs (2 bonding pairs and 2 lone pairs).
- Determine the steric number: The steric number is 4.
- Apply the VSEPR theory: With a steric number of 4, the electron pair arrangement is tetrahedral, and the molecular geometry is bent or V-shape.
Answer: Bent or V-shape
Table of Common Molecular Geometries
| Molecular Geometry | Number of Electron Pairs | Example Molecules |
|---|---|---|
| Linear | 2 | CO2, HCN |
| Trigonal Planar | 3 | BCl3, SO3 |
| Tetrahedral | 4 | CH4, NH3 |
| Trigonal Bipyramidal | 5 | PCl5, SbCl5 |
| Octahedral | 6 | SF6, XeF6 |
Important Notes
👀 Note: The VSEPR theory assumes that all electron pairs are equally spaced and that the central atom is in the center of the molecule.
💡 Note: Molecular geometry is a three-dimensional concept, so it's essential to visualize the molecule in 3D to understand its shape.
What is the VSEPR theory?
+The VSEPR theory states that electron pairs in the valence shell of an atom repel each other and arrange themselves to minimize their mutual repulsion.
How do I determine the molecular geometry of a molecule?
+To determine the molecular geometry of a molecule, draw the Lewis structure, identify the central atom, count the electron pairs, determine the steric number, and apply the VSEPR theory.
What is the difference between electron pairs and steric number?
+Electron pairs include both bonding pairs (shared pairs) and lone pairs (unshared pairs), while the steric number is the total number of electron pairs on the central atom.
Understanding molecular geometry is essential in chemistry, as it helps us predict the shape and structure of molecules, which in turn affects their physical and chemical properties. By mastering the VSEPR theory and practicing with different molecules, you’ll become proficient in determining the molecular geometry of various molecules.
Related Terms:
- Molecular geometry and VSEPR Worksheet
- Molecular geometry chart
- Shapes of molecules Worksheet Answers
- VSEPR Worksheet high school pdf
- VSEPR Practice Problems pdf