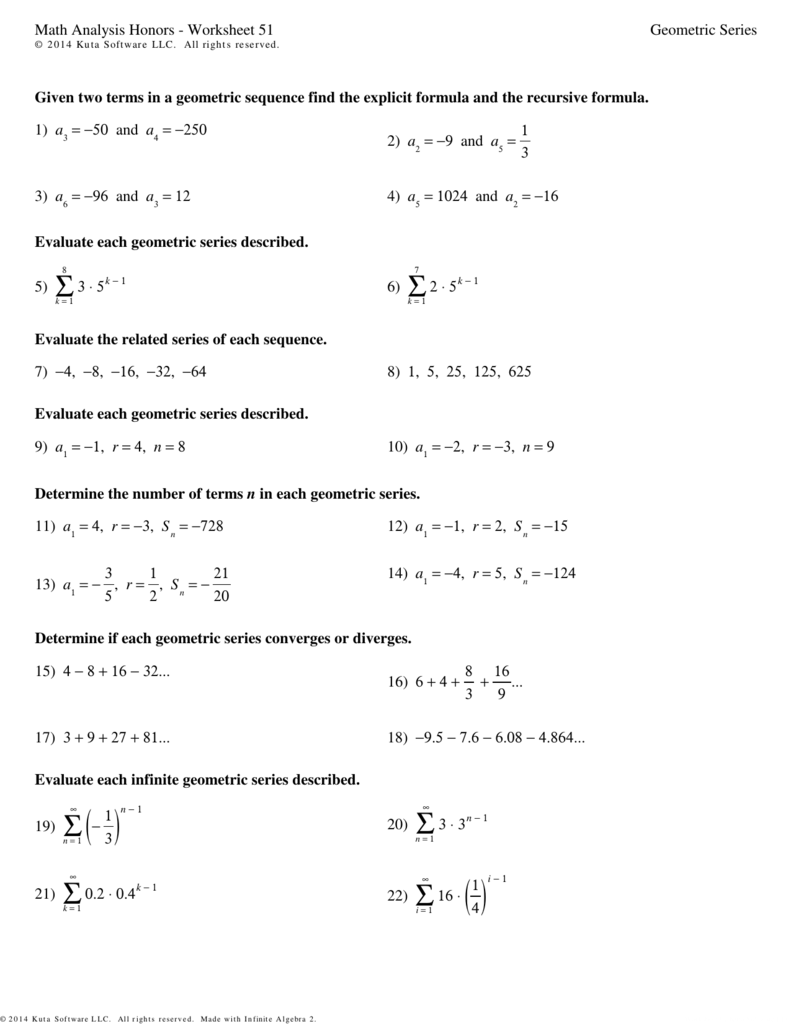
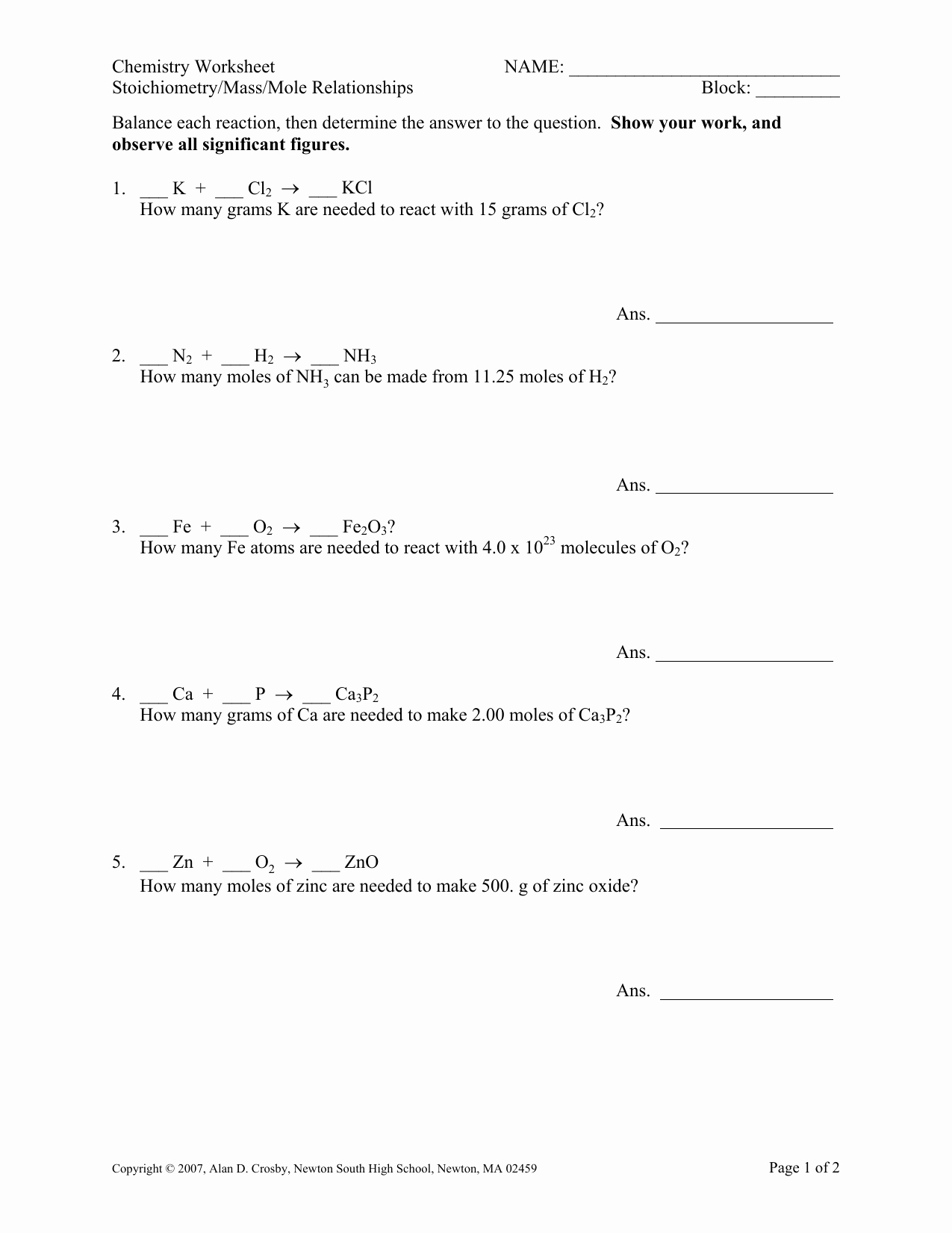
6 Ways to Master Mole Worksheet Chemistry Answers

Understanding Mole Worksheet Chemistry Answers
Mastering mole worksheet chemistry answers is a crucial aspect of chemistry, as it enables students to understand the fundamental principles of chemistry, including the mole concept, stoichiometry, and chemical reactions. In this article, we will explore six ways to master mole worksheet chemistry answers, providing a comprehensive guide for students to improve their understanding of chemistry.
1. Understand the Mole Concept
The mole concept is a fundamental principle in chemistry, and it is essential to understand it to master mole worksheet chemistry answers. A mole is a unit of measurement that represents 6.022 x 10^23 particles, such as atoms or molecules. This concept is used to express the amount of a substance in a chemical reaction.
Key Points to Remember:
- A mole is a unit of measurement that represents 6.022 x 10^23 particles.
- The mole concept is used to express the amount of a substance in a chemical reaction.
- The mole is a fundamental principle in chemistry, and it is essential to understand it to master mole worksheet chemistry answers.
2. Practice Converting between Moles and Grams
Converting between moles and grams is a critical aspect of mastering mole worksheet chemistry answers. This conversion involves using the molar mass of a substance to convert between moles and grams.
Example Problem:
Convert 2.5 moles of carbon dioxide (CO2) to grams.
Solution:
- Determine the molar mass of CO2: 44.01 g/mol
- Multiply the number of moles by the molar mass: 2.5 mol x 44.01 g/mol = 110.025 g
Key Points to Remember:
- Converting between moles and grams involves using the molar mass of a substance.
- The molar mass is used to convert between moles and grams.
3. Master Stoichiometry
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. Mastering stoichiometry is essential to mastering mole worksheet chemistry answers.
Example Problem:
Determine the number of moles of oxygen (O2) required to react with 2.5 moles of hydrogen (H2) to form water (H2O).
Solution:
- Write the balanced equation: 2H2 + O2 → 2H2O
- Determine the mole ratio of O2 to H2: 1 mole O2 : 2 moles H2
- Multiply the number of moles of H2 by the mole ratio: 2.5 mol H2 x (1 mol O2 / 2 mol H2) = 1.25 mol O2
Key Points to Remember:
- Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions.
- Mastering stoichiometry is essential to mastering mole worksheet chemistry answers.
4. Use the Mole Map
The mole map is a diagram that illustrates the relationships between moles, grams, and particles. Using the mole map can help students to visualize the relationships between these quantities and master mole worksheet chemistry answers.
| Moles | Grams | Particles |
|---|---|---|
| 1 mole | molar mass (g) | 6.022 x 10^23 particles |
Key Points to Remember:
- The mole map is a diagram that illustrates the relationships between moles, grams, and particles.
- Using the mole map can help students to visualize the relationships between these quantities.
5. Practice, Practice, Practice
Practicing mole worksheet chemistry answers is essential to mastering the mole concept and stoichiometry. Students should practice converting between moles and grams, mastering stoichiometry, and using the mole map to visualize the relationships between moles, grams, and particles.
Example Problems:
- Convert 3.5 moles of sodium chloride (NaCl) to grams.
- Determine the number of moles of oxygen (O2) required to react with 1.5 moles of hydrogen (H2) to form water (H2O).
Key Points to Remember:
- Practicing mole worksheet chemistry answers is essential to mastering the mole concept and stoichiometry.
- Students should practice converting between moles and grams, mastering stoichiometry, and using the mole map.
6. Review and Reflect
Reviewing and reflecting on mole worksheet chemistry answers is essential to mastering the mole concept and stoichiometry. Students should review the key points and reflect on their understanding of the material.
Key Points to Remember:
- Reviewing and reflecting on mole worksheet chemistry answers is essential to mastering the mole concept and stoichiometry.
- Students should review the key points and reflect on their understanding of the material.
📝 Note: Mastering mole worksheet chemistry answers requires practice, patience, and persistence. Students should review the key points and reflect on their understanding of the material to ensure mastery.
Mastering mole worksheet chemistry answers is a challenging task, but with practice, patience, and persistence, students can develop a deep understanding of the mole concept and stoichiometry. By following these six steps, students can improve their understanding of chemistry and become proficient in mole worksheet chemistry answers.
What is the mole concept?
+The mole concept is a fundamental principle in chemistry that represents 6.022 x 10^23 particles, such as atoms or molecules.
How do I convert between moles and grams?
+To convert between moles and grams, use the molar mass of a substance to convert between moles and grams.
What is stoichiometry?
+Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions.
Related Terms:
- Mole Problems Worksheet with answers
- Mole Calculation Worksheet pdf
- Moles Calculations GCSE Worksheet PDF
- Mole Calculation Worksheet 1
- worksheet mole/mass problems