Convert Mole to Grams and Grams to Moles Easily
Converting Between Moles and Grams: A Comprehensive Guide
When working with chemical reactions, it’s often necessary to convert between moles and grams. This can be a challenging task, especially for those who are new to chemistry. In this article, we’ll provide a step-by-step guide on how to convert moles to grams and grams to moles easily.
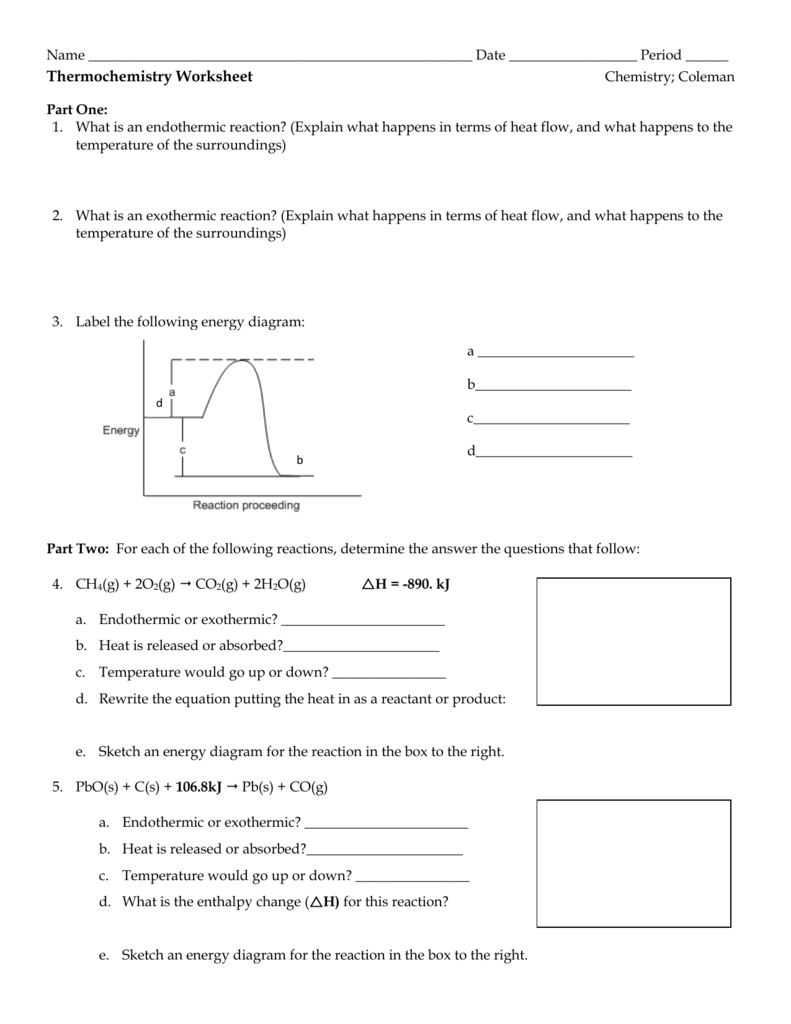
Understanding the Basics: Moles and Grams
Before we dive into the conversion process, let’s quickly review the basics.
- A mole (mol) is a unit of measurement that represents 6.022 x 10^23 particles (atoms or molecules).
- A gram (g) is a unit of measurement that represents the mass of a substance.
Converting Moles to Grams
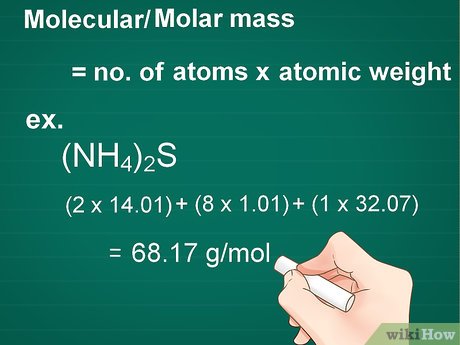
To convert moles to grams, you need to know the molar mass of the substance. The molar mass is the mass of one mole of a substance, usually expressed in grams per mole (g/mol).
Here’s the step-by-step process:
- Look up the molar mass of the substance you’re working with.
- Multiply the number of moles by the molar mass.
Formula: moles x molar mass = mass in grams
Example:
- Convert 2 moles of sodium chloride (NaCl) to grams.
- Molar mass of NaCl = 58.44 g/mol
- Mass in grams = 2 mol x 58.44 g/mol = 116.88 g
📝 Note: Make sure to check the units of the molar mass to ensure they match the units of the mass you're trying to convert to.
Converting Grams to Moles
To convert grams to moles, you need to know the molar mass of the substance.
Here’s the step-by-step process:
- Look up the molar mass of the substance you’re working with.
- Divide the mass in grams by the molar mass.
Formula: mass in grams ÷ molar mass = moles
Example:
- Convert 100 grams of calcium carbonate (CaCO3) to moles.
- Molar mass of CaCO3 = 100.09 g/mol
- Moles = 100 g ÷ 100.09 g/mol = 0.998 mol
📝 Note: Make sure to check the units of the molar mass to ensure they match the units of the mass you're trying to convert from.
Using Conversion Factors
Another way to convert between moles and grams is to use conversion factors. A conversion factor is a ratio of two units that are equal to each other.
Here’s how to use conversion factors:
- Write down the given information as a ratio (e.g., moles : grams).
- Use the conversion factor to convert to the desired unit.
Example:
- Convert 2 moles of sodium chloride (NaCl) to grams using conversion factors.
- Molar mass of NaCl = 58.44 g/mol
- Conversion factor: 1 mol NaCl = 58.44 g
- Mass in grams = 2 mol x (58.44 g / 1 mol) = 116.88 g
Common Conversion Factors
Here are some common conversion factors that you may find useful:

| Substance | Molar Mass (g/mol) | Conversion Factor |
|---|---|---|
| Sodium chloride (NaCl) | 58.44 | 1 mol NaCl = 58.44 g |
| Calcium carbonate (CaCO3) | 100.09 | 1 mol CaCO3 = 100.09 g |
| Glucose (C6H12O6) | 180.16 | 1 mol glucose = 180.16 g |
Conclusion
Converting between moles and grams is a crucial skill in chemistry. By understanding the basics of moles and grams, and using the formulas and conversion factors outlined in this article, you’ll be able to easily convert between these two units.
Remember to always check the units of the molar mass to ensure they match the units of the mass you’re trying to convert to or from.
What is the difference between a mole and a gram?
+A mole is a unit of measurement that represents 6.022 x 10^23 particles (atoms or molecules), while a gram is a unit of measurement that represents the mass of a substance.
How do I convert moles to grams?
+To convert moles to grams, multiply the number of moles by the molar mass of the substance.
What is the molar mass of a substance?
+The molar mass of a substance is the mass of one mole of that substance, usually expressed in grams per mole (g/mol).