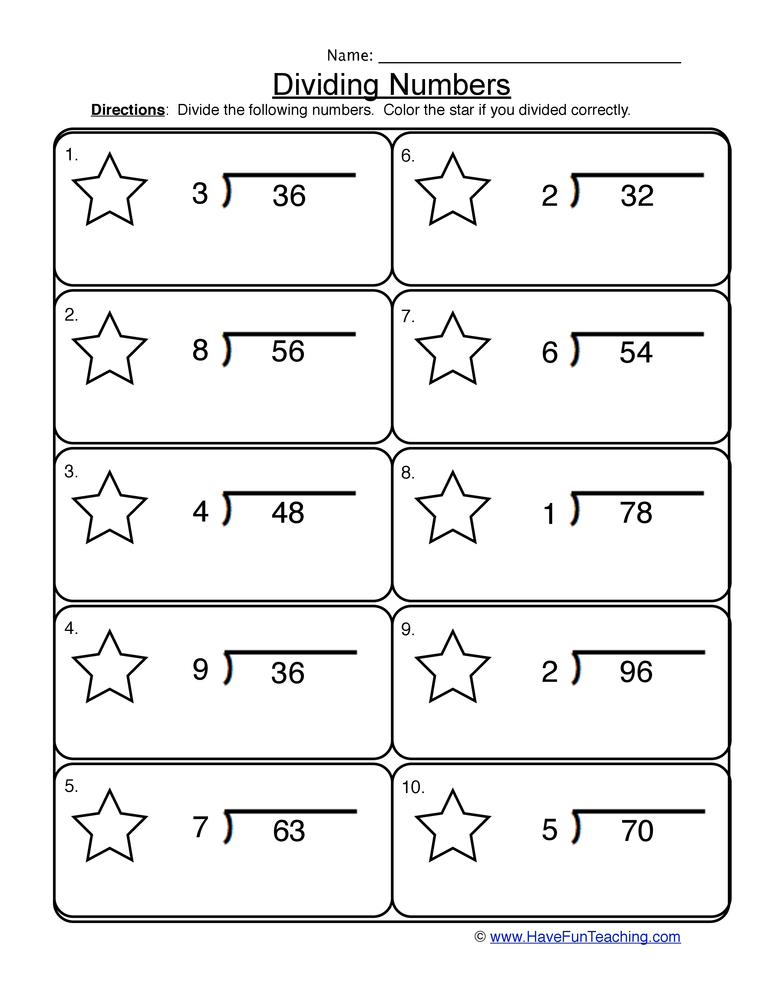
Mole Particle Conversions Worksheet Made Easy
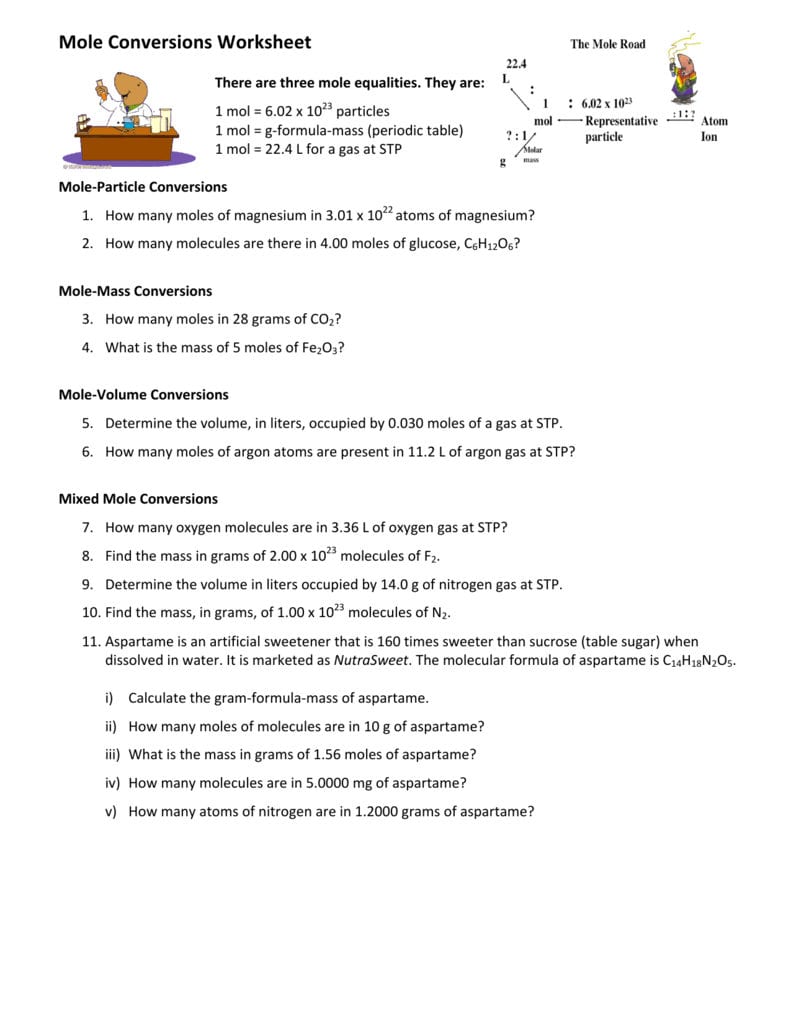
Mole Particle Conversions Worksheet Made Easy
Particle conversions are an essential concept in chemistry that deals with the relationship between moles and particles of a substance. This worksheet is designed to help you master particle conversions with ease. In this comprehensive guide, we will break down the steps and provide examples to make particle conversions a breeze.
Understanding the Basics
Before diving into particle conversions, it’s crucial to understand the fundamental concepts:
- Atoms: The building blocks of matter.
- Molecules: A group of two or more atoms bonded together.
- Moles: A unit of measurement representing 6.022 x 10^23 particles (atoms or molecules).
Mole-Particle Conversion Steps
To perform particle conversions, follow these straightforward steps:
- Identify the given information: Determine the number of moles or particles of a substance.
- Determine the conversion factor: Use Avogadro’s number (6.022 x 10^23 particles/mol) to convert between moles and particles.
- Apply the conversion factor: Multiply or divide the given information by the conversion factor to obtain the desired units.
Examples and Solutions
Let’s practice particle conversions with some examples:
Example 1: Convert 2.5 moles of oxygen molecules (O2) to particles.
- Given: 2.5 mol O2
- Conversion factor: 6.022 x 10^23 particles/mol
- Solution: 2.5 mol O2 x (6.022 x 10^23 particles/mol) = 1.5055 x 10^24 particles
Example 2: Convert 3.2 x 10^22 particles of carbon dioxide (CO2) to moles.
- Given: 3.2 x 10^22 particles CO2
- Conversion factor: 1 mol / (6.022 x 10^23 particles)
- Solution: 3.2 x 10^22 particles CO2 x (1 mol / (6.022 x 10^23 particles)) = 0.0531 mol
Common Particle Conversions
Here’s a summary of common particle conversions:

| Conversion | Formula |
|---|---|
| Moles to particles | particles = moles x (6.022 x 10^23 particles/mol) |
| Particles to moles | moles = particles / (6.022 x 10^23 particles/mol) |
💡 Note: When converting between moles and particles, make sure to use the correct conversion factor and units.
Conclusion
Mastering particle conversions is a fundamental skill in chemistry. By following the simple steps outlined in this worksheet, you’ll be able to tackle particle conversions with confidence. Remember to practice with different examples and scenarios to reinforce your understanding.
What is Avogadro’s number?
+Avogadro’s number is 6.022 x 10^23 particles/mol, which represents the number of particles in one mole of a substance.
How do I convert moles to particles?
+To convert moles to particles, multiply the number of moles by Avogadro’s number (6.022 x 10^23 particles/mol).
What is the difference between atoms and molecules?
+Atoms are the building blocks of matter, while molecules are groups of two or more atoms bonded together.
Related Terms:
- Mole conversion Worksheet pdf
- gram/mole conversion worksheet and answers
- mass-mole conversions worksheet answer key
- Worksheet mole Conversions
- Mole to Mole Conversion Worksheet
- Mole Conversion Worksheet Mixed Practice