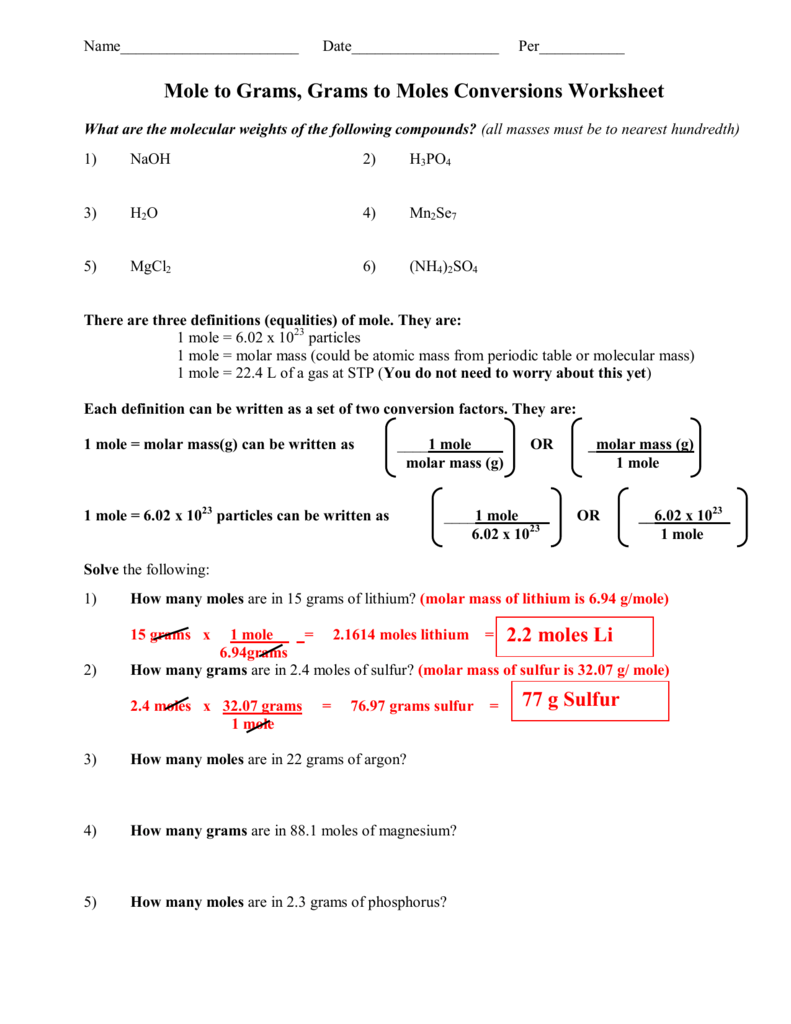
Mole Calculation Practice Worksheet
Mastering Mole Calculations: A Comprehensive Guide and Practice Worksheet
Mole calculations are a fundamental concept in chemistry, and mastering them is essential for any student or professional in the field. In this article, we will delve into the world of mole calculations, explore the concept of the mole, and provide a practice worksheet to help you reinforce your understanding.
What is a Mole?
A mole (mol) is the unit of measurement for the amount of a substance. It is defined as the amount of a substance that contains as many particles (atoms, molecules, or ions) as there are atoms in 0.012 kilograms of carbon-12. This number is known as the Avogadro’s number, which is approximately 6.022 x 10^23 particles.
Understanding Mole Calculations
Mole calculations involve converting between the number of particles (atoms, molecules, or ions) and the amount of a substance in moles. There are several types of mole calculations, including:
- Mole-to-particle calculations: These involve converting the number of moles to the number of particles.
- Particle-to-mole calculations: These involve converting the number of particles to the number of moles.
- Mole-to-mass calculations: These involve converting the number of moles to the mass of a substance.
- Mass-to-mole calculations: These involve converting the mass of a substance to the number of moles.
Practice Worksheet
Now that we have covered the basics of mole calculations, it’s time to practice! Here are 10 problems to help you reinforce your understanding.

| Problem | Answer |
|---|---|
| 1. If you have 3.5 mol of oxygen gas, how many oxygen molecules do you have? | 3.5 mol x 6.022 x 10^23 molecules/mol = 2.11 x 10^24 molecules |
| 2. How many moles of hydrogen ions are present in 0.5 liters of a 2 M HCl solution? | 0.5 liters x 2 M x 6.022 x 10^23 ions/mol = 6.022 x 10^23 ions / 2 = 3.01 x 10^23 ions |
| 3. What is the mass of 2.5 mol of sodium chloride? | 2.5 mol x 58.44 g/mol = 146.1 g |
| 4. If you have 2.2 g of carbon, how many moles of carbon do you have? | 2.2 g / 12.01 g/mol = 0.183 mol |
| 5. How many moles of nitrogen molecules are present in 0.75 liters of air at STP? | 0.75 liters x 0.79 (N2/air) x 6.022 x 10^23 molecules/mol = 3.55 x 10^23 molecules |
| 6. What is the number of oxygen atoms present in 1.5 mol of water? | 1.5 mol x 6.022 x 10^23 molecules/mol x 1 (O2/H2O) = 9.033 x 10^23 molecules |
| 7. If you have 0.35 mol of calcium ions, how many calcium atoms do you have? | 0.35 mol x 6.022 x 10^23 atoms/mol = 2.11 x 10^23 atoms |
| 8. What is the mass of 1.8 mol of ammonia? | 1.8 mol x 17.03 g/mol = 30.65 g |
| 9. How many moles of hydroxide ions are present in 0.25 liters of a 1.5 M NaOH solution? | 0.25 liters x 1.5 M x 6.022 x 10^23 ions/mol = 2.25 x 10^23 ions |
| 10. If you have 4.5 g of iron, how many moles of iron do you have? | 4.5 g / 55.85 g/mol = 0.081 mol |
Notes
📝 Note: When performing mole calculations, always make sure to check your units and ensure that you are using the correct number of significant figures.
Key Takeaways
In conclusion, mole calculations are a crucial part of chemistry, and mastering them is essential for any student or professional in the field. By understanding the concept of the mole and practicing different types of mole calculations, you can become proficient in converting between the number of particles and the amount of a substance in moles.
What is the Avogadro’s number?
+The Avogadro’s number is approximately 6.022 x 10^23 particles.
What are the different types of mole calculations?
+The different types of mole calculations include mole-to-particle calculations, particle-to-mole calculations, mole-to-mass calculations, and mass-to-mole calculations.
Why is it important to check units when performing mole calculations?
+It is essential to check units when performing mole calculations to ensure accuracy and avoid errors.
Related Terms:
- Mole Calculation Practice Worksheet
- Mole Calculation Worksheet answer key
- Worksheet Mole concept
- Mole to mole practice worksheet
- Mass and the mole Worksheet
- Moles Calculations GCSE Worksheet PDF