Molarity Worksheet Answers

Molarity Worksheet Answers
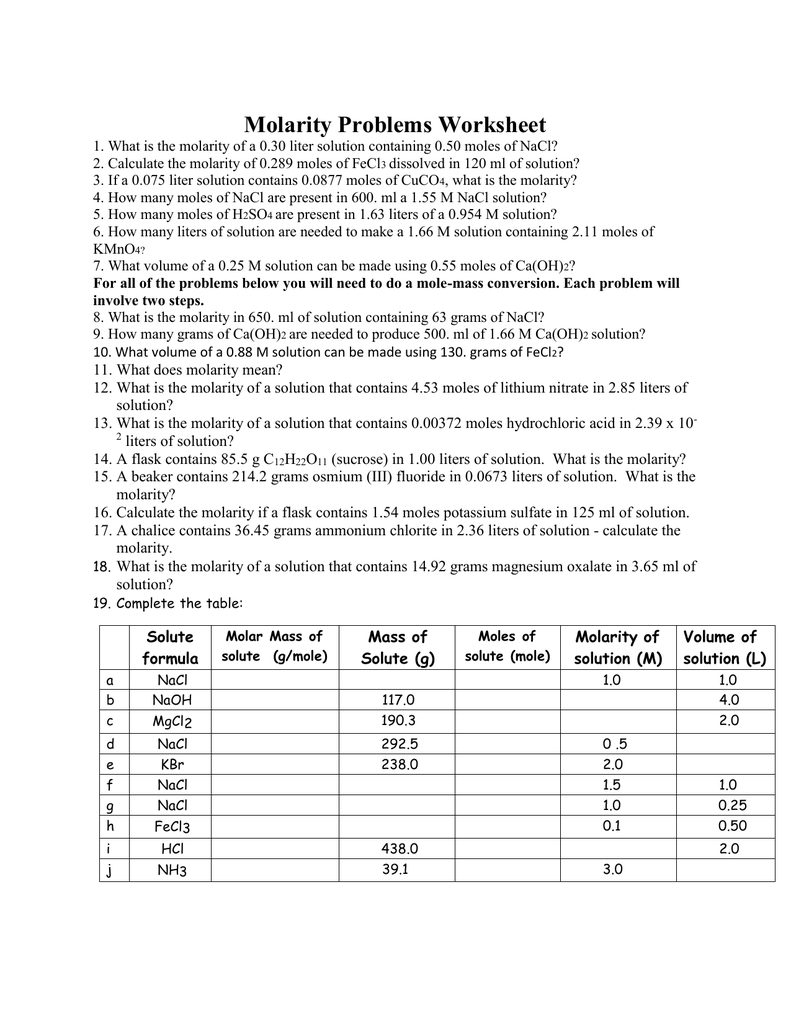
In chemistry, molarity is a fundamental concept that measures the concentration of a solution. It is defined as the number of moles of solute per liter of solution. Here, we will provide answers to a molarity worksheet, explaining the steps and calculations involved in solving each problem.
Problem 1: Calculate the molarity of a solution that contains 25.0 grams of sodium chloride (NaCl) in 500.0 mL of water.
Step 1: Determine the molar mass of NaCl. The molar mass of NaCl is 58.44 g/mol.
Step 2: Calculate the number of moles of NaCl. moles = mass of NaCl / molar mass of NaCl = 25.0 g / 58.44 g/mol = 0.428 mol
Step 3: Calculate the volume of the solution in liters. volume = 500.0 mL / 1000 mL/L = 0.5 L
Step 4: Calculate the molarity of the solution. molarity = moles of NaCl / volume of solution = 0.428 mol / 0.5 L = 0.856 M
Answer: The molarity of the solution is 0.856 M.
Problem 2: Calculate the molarity of a solution that contains 15.0 grams of glucose (C6H12O6) in 250.0 mL of water.
Step 1: Determine the molar mass of C6H12O6. The molar mass of C6H12O6 is 180.16 g/mol.
Step 2: Calculate the number of moles of C6H12O6. moles = mass of C6H12O6 / molar mass of C6H12O6 = 15.0 g / 180.16 g/mol = 0.083 mol
Step 3: Calculate the volume of the solution in liters. volume = 250.0 mL / 1000 mL/L = 0.25 L
Step 4: Calculate the molarity of the solution. molarity = moles of C6H12O6 / volume of solution = 0.083 mol / 0.25 L = 0.332 M
Answer: The molarity of the solution is 0.332 M.
Problem 3: A solution contains 20.0 grams of calcium chloride (CaCl2) in 750.0 mL of water. Calculate the molarity of the solution.
Step 1: Determine the molar mass of CaCl2. The molar mass of CaCl2 is 110.98 g/mol.
Step 2: Calculate the number of moles of CaCl2. moles = mass of CaCl2 / molar mass of CaCl2 = 20.0 g / 110.98 g/mol = 0.180 mol
Step 3: Calculate the volume of the solution in liters. volume = 750.0 mL / 1000 mL/L = 0.75 L
Step 4: Calculate the molarity of the solution. molarity = moles of CaCl2 / volume of solution = 0.180 mol / 0.75 L = 0.240 M
Answer: The molarity of the solution is 0.240 M.
Problem 4: A 0.50 M solution of potassium nitrate (KNO3) has a volume of 200.0 mL. Calculate the mass of KNO3 present in the solution.
Step 1: Determine the molar mass of KNO3. The molar mass of KNO3 is 101.10 g/mol.
Step 2: Calculate the number of moles of KNO3. moles = molarity x volume = 0.50 M x 0.2 L = 0.10 mol
Step 3: Calculate the mass of KNO3. mass = moles x molar mass = 0.10 mol x 101.10 g/mol = 10.11 g
Answer: The mass of KNO3 present in the solution is 10.11 g.
Notes:
- When calculating molarity, always express the volume in liters (L).
- The molar mass of a compound is the sum of the atomic masses of its constituent elements.
- When solving problems, always label your units and show your work.
Molarity Conversion Table:
| Molarity | Moles/Liter | Moles/1000 mL |
|---|---|---|
| 1 M | 1 mol/L | 1 mol/1000 mL |
| 0.1 M | 0.1 mol/L | 0.1 mol/1000 mL |
| 0.01 M | 0.01 mol/L | 0.01 mol/1000 mL |
Remember, molarity is a measure of concentration, so it’s essential to use the correct units when expressing molarity.
To wrap up this worksheet, we hope you now have a better understanding of how to calculate molarity and solve problems related to it. Practice makes perfect, so be sure to try more problems on your own to reinforce your knowledge.
What is molarity?
+
Molarity is a measure of the concentration of a solution, defined as the number of moles of solute per liter of solution.
How do I calculate molarity?
+
To calculate molarity, divide the number of moles of solute by the volume of the solution in liters.
What is the difference between molarity and molality?
+
Molarity is the number of moles of solute per liter of solution, while molality is the number of moles of solute per kilogram of solvent.