5 Tips for Mastering Covalent Bonding Worksheets
Covalent Bonding: Understanding the Basics
Covalent bonding is a fundamental concept in chemistry that describes the sharing of electrons between atoms to form a chemical bond. It’s a crucial topic in chemistry, and mastering covalent bonding worksheets is essential for students to succeed in their chemistry studies. In this article, we’ll provide you with 5 tips to help you master covalent bonding worksheets and become a chemistry whiz.
Tip 1: Understand the Octet Rule
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer energy level, which typically consists of eight electrons. This rule is crucial in understanding covalent bonding, as it helps you determine the number of electrons that an atom will share with other atoms. Make sure you understand the octet rule before moving on to covalent bonding worksheets.
To apply the octet rule, follow these steps:
- Identify the atoms involved in the bond
- Determine the number of valence electrons for each atom
- Calculate the number of electrons needed to achieve a full outer energy level
- Draw the Lewis structure to visualize the bond
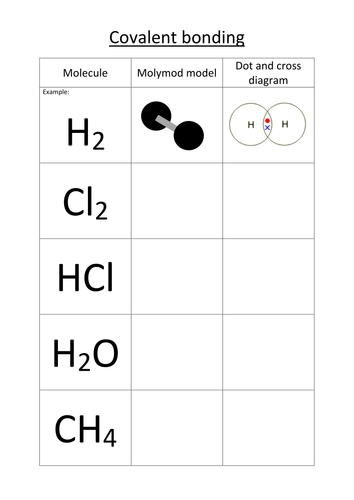
Tip 2: Draw Lewis Structures Correctly
Lewis structures are a crucial part of covalent bonding worksheets. They help you visualize the bond and determine the number of electrons shared between atoms. To draw Lewis structures correctly, follow these steps:
- Determine the central atom
- Calculate the total number of valence electrons
- Draw single bonds between the central atom and surrounding atoms
- Add electrons to the surrounding atoms to satisfy the octet rule
- Use double or triple bonds if necessary to satisfy the octet rule
💡 Note: Make sure to use the correct number of electrons and follow the octet rule when drawing Lewis structures.
Tip 3: Identify the Type of Covalent Bond
There are two main types of covalent bonds: polar and nonpolar. Polar covalent bonds involve the unequal sharing of electrons, resulting in a dipole moment. Nonpolar covalent bonds involve the equal sharing of electrons. To identify the type of covalent bond, follow these steps:
- Determine the electronegativity of the atoms involved
- Calculate the difference in electronegativity
- If the difference is 0.5 or greater, the bond is polar
- If the difference is less than 0.5, the bond is nonpolar
Tip 4: Practice, Practice, Practice
Practice is key to mastering covalent bonding worksheets. The more you practice, the more comfortable you’ll become with drawing Lewis structures and identifying the type of covalent bond. Here are some tips to help you practice effectively:
- Start with simple molecules, such as H2 and O2
- Gradually move on to more complex molecules, such as CO2 and CH4
- Use online resources, such as worksheets and quizzes, to practice
- Join a study group or find a study partner to practice with
Tip 5: Use Online Resources
There are many online resources available to help you master covalent bonding worksheets. Here are some of our favorites:
- Khan Academy: Offers video tutorials and practice exercises on covalent bonding
- Crash Course: Offers video tutorials and practice exercises on covalent bonding
- Chemistry LibreTexts: Offers online textbooks and practice exercises on covalent bonding
| Resource | Description |
|---|---|
| Khan Academy | Video tutorials and practice exercises on covalent bonding |
| Crash Course | Video tutorials and practice exercises on covalent bonding |
| Chemistry LibreTexts | Online textbooks and practice exercises on covalent bonding |
By following these 5 tips, you’ll be well on your way to mastering covalent bonding worksheets. Remember to practice regularly, use online resources, and seek help when needed. Good luck!
As you continue to study covalent bonding, remember that it’s a fundamental concept in chemistry that will help you understand more complex topics. By mastering covalent bonding worksheets, you’ll be able to tackle more challenging topics with confidence.
What is the octet rule?
+The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer energy level, which typically consists of eight electrons.
What is a Lewis structure?
+A Lewis structure is a diagram that shows the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
What is the difference between a polar and nonpolar covalent bond?
+A polar covalent bond involves the unequal sharing of electrons, resulting in a dipole moment. A nonpolar covalent bond involves the equal sharing of electrons.