5 Ways to Master Molarity by Dilution
Understanding Molarity and Dilution
Molarity is a fundamental concept in chemistry that measures the concentration of a solution. It is defined as the number of moles of solute per liter of solution. Dilution is the process of reducing the concentration of a solution by adding more solvent. Mastering molarity by dilution is a crucial skill for chemists, researchers, and students to accurately prepare and manipulate solutions.
Method 1: Calculating Molarity by Dilution
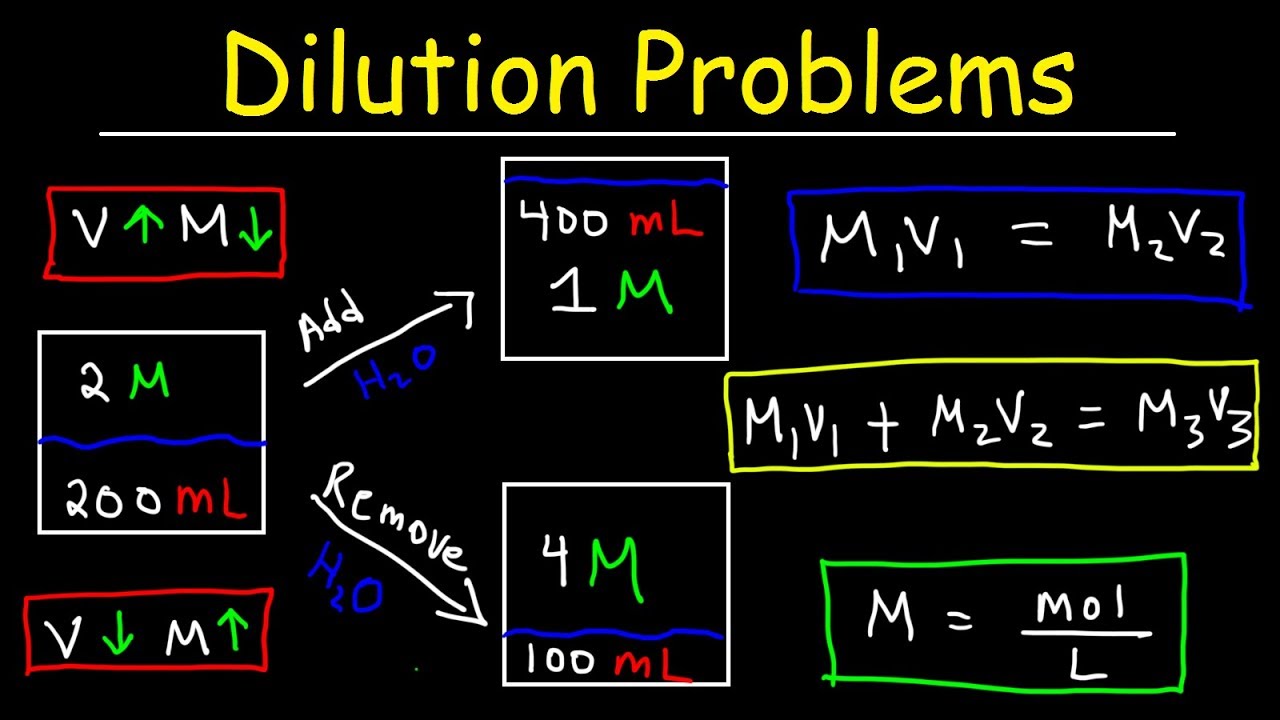
To calculate molarity by dilution, you need to know the initial molarity, volume, and final volume of the solution. The formula for calculating molarity by dilution is:
M1V1 = M2V2
Where: M1 = initial molarity V1 = initial volume M2 = final molarity V2 = final volume
For example, if you have 100 mL of a 2M solution and you want to dilute it to 500 mL, the final molarity would be:
M1V1 = M2V2 2M x 100mL = M2 x 500mL M2 = (2M x 100mL) / 500mL M2 = 0.4M
🔍 Note: When calculating molarity by dilution, it's essential to use the correct units for volume (mL or L) and molarity (M).
Method 2: Using a Dilution Table
A dilution table is a convenient tool for calculating molarity by dilution. The table provides a series of dilution factors that can be used to calculate the final molarity.
| Dilution Factor | Initial Volume (mL) | Final Volume (mL) | Final Molarity (M) |
|---|---|---|---|
| 1:1 | 100 | 200 | 0.5M |
| 1:2 | 100 | 300 | 0.33M |
| 1:5 | 100 | 600 | 0.17M |
| … | … | … | … |
Using the dilution table, you can quickly determine the final molarity by finding the corresponding dilution factor.
Method 3: Measuring Volumes Using a Pipette
Accurate measurement of volumes is critical when preparing solutions. Using a pipette is a precise way to measure volumes, especially when working with small quantities.
When using a pipette, make sure to:
- Use the correct pipette tip size for the volume being measured
- Hold the pipette at a 45-degree angle to avoid air bubbles
- Release the liquid slowly to avoid splashing
Method 4: Serial Dilution
Serial dilution is a method of dilution where a series of dilutions are performed in a stepwise manner. This method is useful when preparing a range of concentrations from a single stock solution.
For example, to prepare a series of concentrations from 1M to 0.01M, you can perform the following serial dilutions:
- Dilute 1M solution to 0.1M (1:10 dilution)
- Dilute 0.1M solution to 0.01M (1:10 dilution)
🔍 Note: When performing serial dilutions, it's essential to accurately record each dilution step to ensure accurate final concentrations.
Method 5: Using a Dilution Calculator
A dilution calculator is an online tool that can simplify the calculation of molarity by dilution. These calculators can be found online and can be used to calculate final molarity, volume, or dilution factor.
Using a dilution calculator can save time and reduce errors, especially when working with complex dilution problems.
When using a dilution calculator, make sure to:
- Enter the correct values for initial molarity, volume, and final volume
- Select the correct units for volume and molarity
- Verify the calculated results to ensure accuracy
In summary, mastering molarity by dilution requires a combination of theoretical understanding, practical skills, and attention to detail. By using one or more of the methods outlined above, you can accurately prepare and manipulate solutions to achieve the desired concentration.
The key to mastering molarity by dilution is to practice regularly and to use a combination of methods to verify results. With time and practice, you will become proficient in calculating molarity by dilution and will be able to accurately prepare solutions for a variety of applications.
What is the formula for calculating molarity by dilution?
+M1V1 = M2V2
What is the purpose of a dilution table?
+A dilution table is a convenient tool for calculating molarity by dilution. It provides a series of dilution factors that can be used to calculate the final molarity.
What is serial dilution?
+Serial dilution is a method of dilution where a series of dilutions are performed in a stepwise manner. This method is useful when preparing a range of concentrations from a single stock solution.