5 Ways to Master Molarity and Dilution Calculations
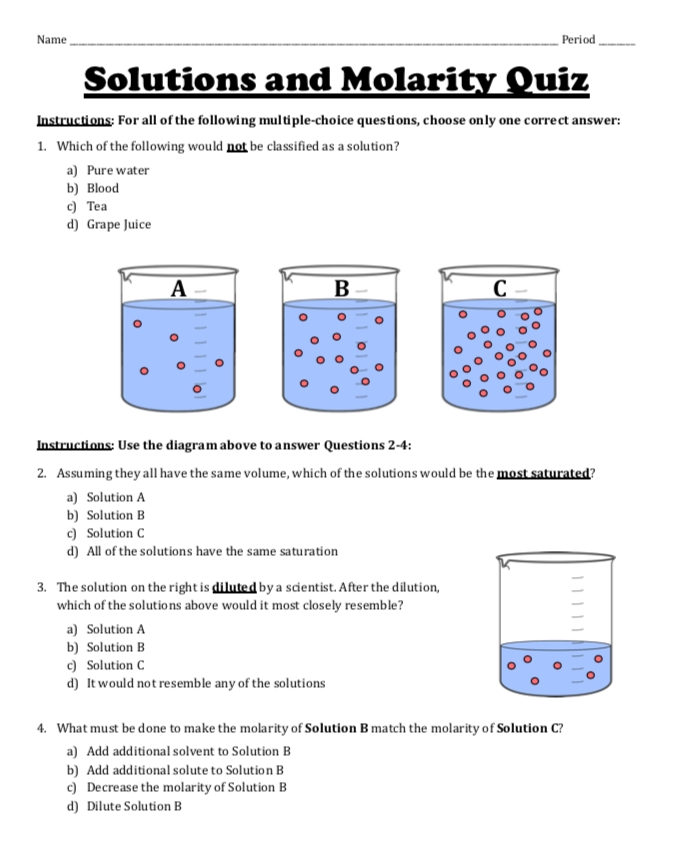
Understanding Molarity and Dilution Calculations
Molarity and dilution calculations are fundamental concepts in chemistry, particularly in laboratory settings. Mastering these calculations is essential for scientists, researchers, and students to ensure accuracy and precision in their experiments. In this article, we will explore five ways to master molarity and dilution calculations, providing you with a comprehensive guide to tackle these complex concepts.
1. Understand the Basics of Molarity
Before diving into calculations, it’s crucial to understand the basics of molarity. Molarity is defined as the number of moles of a substance per liter of solution. It’s expressed in units of moles per liter (mol/L) or molarity (M). To calculate molarity, you need to know the number of moles of the substance and the volume of the solution in liters.
💡 Note: Make sure to use the correct units when calculating molarity, as it can significantly affect the accuracy of your results.
2. Master the Molarity Formula
The molarity formula is a straightforward equation that helps you calculate the molarity of a solution:
Molarity (M) = Number of moles (n) / Volume of solution (V)
Where:
- M = Molarity (mol/L)
- n = Number of moles
- V = Volume of solution (L)
For example, if you have 2 moles of a substance dissolved in 1 liter of solution, the molarity would be:
M = 2 mol / 1 L = 2 M
3. Dilution Calculations: Understanding the Concept
Dilution calculations involve changing the concentration of a solution by adding or removing solvent. There are two types of dilution calculations:
- Concentrating a solution: Adding more solute to a solution to increase its concentration.
- Diluting a solution: Adding more solvent to a solution to decrease its concentration.
To perform dilution calculations, you need to understand the concept of dilution factor, which is the ratio of the initial volume to the final volume.
4. Mastering Dilution Calculations
To master dilution calculations, follow these steps:
- Identify the initial concentration (M1) and volume (V1) of the solution.
- Determine the final concentration (M2) and volume (V2) of the solution.
- Calculate the dilution factor (DF) using the formula:
DF = V2 / V1
- Use the dilution factor to calculate the new concentration (M2) using the formula:
M2 = M1 x DF
For example, if you have a 2 M solution with a volume of 1 liter, and you want to dilute it to 1 M with a final volume of 2 liters, the dilution factor would be:
DF = 2 L / 1 L = 2
The new concentration would be:
M2 = 2 M x 2 = 1 M
5. Practice with Real-World Examples
Practice makes perfect! To master molarity and dilution calculations, practice with real-world examples. Try solving problems with different scenarios, such as:
- Preparing a solution with a specific concentration
- Diluting a solution to a desired concentration
- Concentrating a solution to a higher concentration
Use online resources, such as calculators or worksheets, to practice and reinforce your understanding.
What is the difference between molarity and molality?
+Molarity is the number of moles of a substance per liter of solution, while molality is the number of moles of a substance per kilogram of solvent.
How do I calculate the number of moles of a substance?
+To calculate the number of moles of a substance, use the formula: moles = mass / molar mass.
What is the significance of dilution factor in dilution calculations?
+The dilution factor is the ratio of the initial volume to the final volume, which helps you calculate the new concentration of a solution after dilution.
In conclusion, mastering molarity and dilution calculations requires a solid understanding of the concepts and formulas. By following these five ways, you’ll be able to tackle complex calculations with confidence and accuracy. Remember to practice regularly and apply your knowledge to real-world scenarios to become a master of molarity and dilution calculations.
Related Terms:
- Molarity Worksheet With answers pdf
- Molarity answer Key
- molarity % worksheet pdf
- Making dilutions Worksheet
- Concentrations and Dilutions Worksheet answers