Matter Properties Worksheet
Understanding the Properties of Matter
Matter is anything that has mass and takes up space by having volume. All physical objects in the universe are composed of matter, and it is a fundamental concept in physics and chemistry. In this article, we will explore the properties of matter, which are essential for understanding the behavior of materials and their applications in various fields.
Physical Properties of Matter
Physical properties of matter are characteristics that can be observed or measured without changing the identity of the substance. These properties include:
- Mass: a measure of the amount of matter in an object
- Volume: the amount of space occupied by an object
- Density: the ratio of mass to volume of a substance
- Melting point: the temperature at which a solid changes state to a liquid
- Boiling point: the temperature at which a liquid changes state to a gas
- Color: the visible light reflected by an object
- Odor: the smell of a substance
- Taste: the flavor of a substance
Chemical Properties of Matter
Chemical properties of matter are characteristics that describe the way a substance reacts with other substances. These properties include:
- Flammability: the ability of a substance to burn in the presence of oxygen
- Reactivity: the ability of a substance to react with other substances
- Corrosion: the ability of a substance to deteriorate or wear away due to chemical reactions
- Toxicity: the ability of a substance to cause harm or death
Types of Matter
Matter can be classified into four main types:
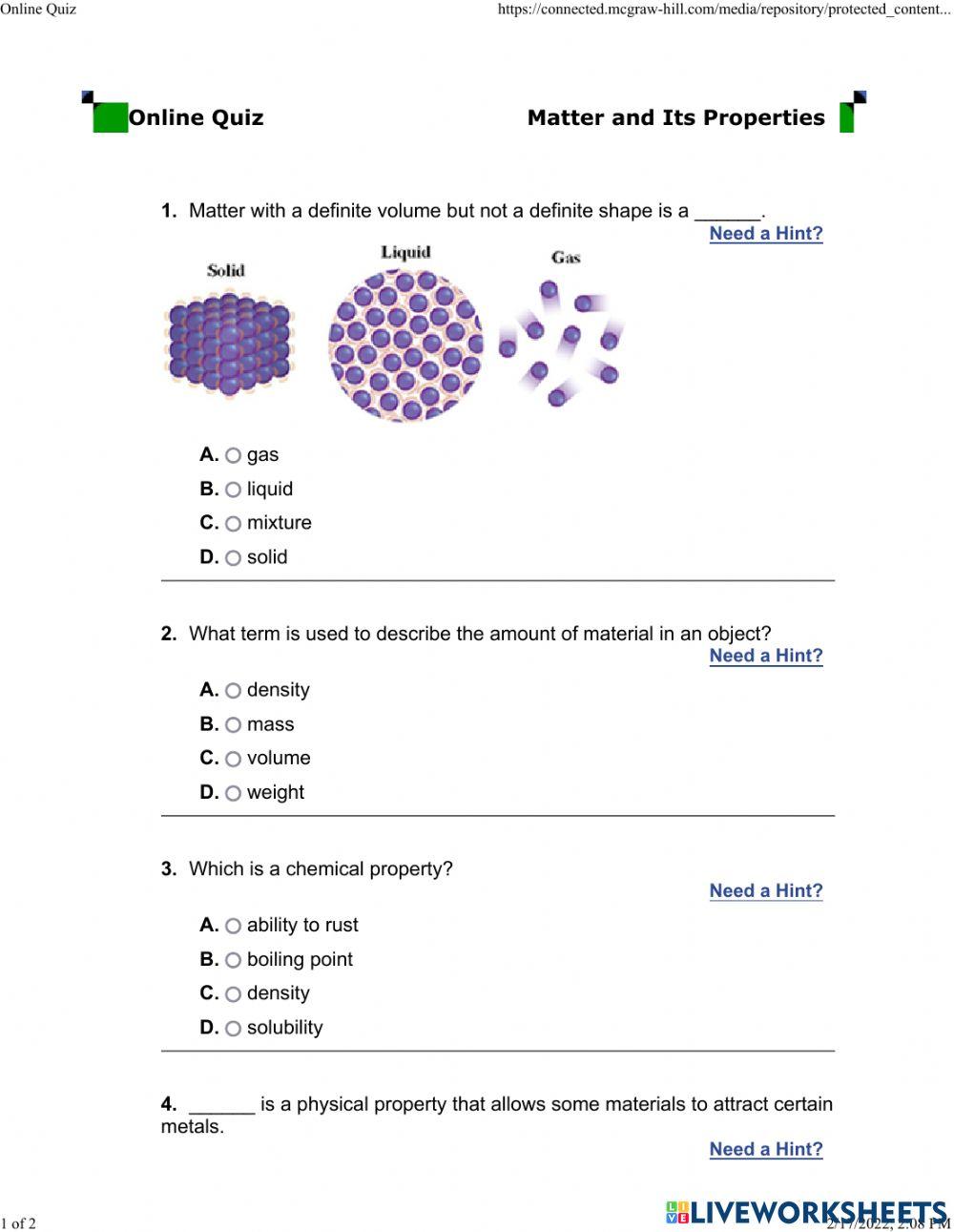
- Solid: a state of matter that has a fixed shape and volume
- Liquid: a state of matter that has a fixed volume but takes the shape of its container
- Gas: a state of matter that has neither a fixed shape nor a fixed volume
- Plasma: a high-energy state of matter that consists of ionized gases
Phase Changes of Matter
Phase changes occur when a substance changes from one state of matter to another. There are six types of phase changes:
- Melting: a solid changes to a liquid
- Freezing: a liquid changes to a solid
- Vaporization: a liquid changes to a gas
- Condensation: a gas changes to a liquid
- Sublimation: a solid changes directly to a gas
- Deposition: a gas changes directly to a solid
🔍 Note: Phase changes are reversible, meaning that a substance can change back to its original state.
Applications of Matter Properties
Understanding the properties of matter is crucial in various fields, including:
- Materials science: the study of the properties and applications of materials
- Chemical engineering: the design and operation of plants that manufacture chemicals and other products
- Aerospace engineering: the design and development of aircraft and spacecraft
- Biomedical engineering: the application of engineering principles to medical devices and systems
Conclusion
In conclusion, the properties of matter are essential for understanding the behavior of materials and their applications in various fields. By studying the physical and chemical properties of matter, we can design and develop new materials and technologies that improve our daily lives.
What is the difference between mass and weight?
+Mass is a measure of the amount of matter in an object, while weight is a measure of the force exerted on an object by gravity.
What is the difference between a physical property and a chemical property?
+A physical property is a characteristic that can be observed or measured without changing the identity of the substance, while a chemical property is a characteristic that describes the way a substance reacts with other substances.
What are the four main types of matter?
+The four main types of matter are solid, liquid, gas, and plasma.
Related Terms:
- Properties of matter worksheet PDF
- Physical properties of matter Worksheet